Allogeneic RPE cell suspension manufactured at scale demonstrating preclinical safety and efficacy led to IND approval
- PMID: 40253438
- PMCID: PMC12009284
- DOI: 10.1038/s41536-025-00407-0
Allogeneic RPE cell suspension manufactured at scale demonstrating preclinical safety and efficacy led to IND approval
Abstract
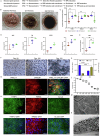
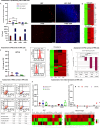
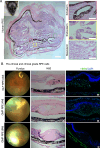
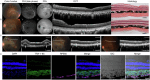
Cell replacement therapy is a promising therapeutic option for dry age-related macular degeneration (AMD). In this study, we outline our design for scalable manufacture with appropriate quality gates and present in vivo data for establishing preclinical safety and efficacy of an induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) product, thus laying the foundation for Phase 1/2a trial approval in India (ClinicalTrials.gov ID: NCT06394232; date of registration: 23rd September 2024). Escalating doses of RPE cell suspension in immunocompromised animals demonstrated absence of tumor formation up to 9 months post-injection. Good Laboratory Practices (GLP) toxicology and tolerability studies in rabbits and non-human primates (NHP) respectively showed no major adverse events. RPE transplanted into immune suppressed RCS rats showed integration, neuroprotection and rescue of visual function. In addition, we provide a detailed description of the modifications in GMP manufacturing protocol to create a final product with a unique composition and Chemistry, Manufacturing and Controls (CMC) studies performed during product development.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Ambati, J., Ambati, B. K., Yoo, S. H., Ianchulev, S. & Adamis, A. P. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv. Ophthalmol.48, 257–293 (2003). - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

