Dual-enzyme activated theranostic nanoparticles for image-guided glioblastoma therapy
- PMID: 40253484
- PMCID: PMC12009400
- DOI: 10.1038/s41598-025-97775-w
Dual-enzyme activated theranostic nanoparticles for image-guided glioblastoma therapy
Abstract
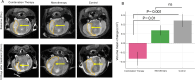
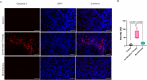
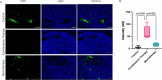
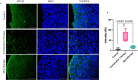
Matrix metalloproteinase-14 (MMP-14) and Cathepsin-B (Cat-B) are overexpressed in glioblastoma (GBM) and not normal brain, making them promising targets for prodrug activation. We investigated a novel combination therapy using two tumor-enzyme activatable theranostic nanoprobes (TNP): TNP-MMP-14, which disrupts the blood tumor barrier via MMP-14 activation, and TNP-Cat-B, which selectively targets GBM cells through Cat-B activation. We hypothesized that combining TNP-MMP-14 and TNP-Cat-B would enhance TNP tumor accumulation and therapeutic efficacy compared to TNP-Cat-B monotherapy. Thirty NSG mice with luciferase-expressing GBM39 tumors received either TNP-MMP-14 plus TNP-Cat-B, TNP-Cat-B only, or saline. Magnetic resonance imaging (MRI) was conducted pre- and post-treatment, with T2* relaxation times analyzed using a generalized linear model. Histopathological differences were assessed using Kruskal-Wallis and Mann-Whitney tests. A Bonferroni correction was applied to account for multiple comparisons. Combination therapy significantly reduced tumor T2* relaxation times (12.98 ± 4.20 ms) compared to TNP-Cat-B monotherapy (22.49 ± 3.95 ms, p < 0.001). The apoptotic marker caspase-3 was also significantly higher in the combination group (64.46 ± 23.43 vs. 15.93 ± 5.81, p < 0.001). These findings demonstrate the potential of dual-enzyme activatable nanoparticles to enhance GBM treatment by overcoming drug delivery barriers and improving therapeutic efficacy over monotherapy.
Keywords: Ferumoxytol; Glioblastoma; MRI; Nanoparticles; Theranostic.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: All experimental procedures involving mice were approved by the Stanford University Administrative Panel on Laboratory Animal Care (Protocol 12040).
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

