Canadian Consensus Guidelines for the Management of Vitiligo
- PMID: 40253664
- PMCID: PMC12092322
- DOI: 10.1007/s13555-025-01402-5
Canadian Consensus Guidelines for the Management of Vitiligo
Abstract
Introduction: Vitiligo remains a highly burdensome disease associated with significant autoimmune and psychosocial comorbidities. Although the therapeutic landscape has long been dominated by off-label therapy, new treatments are emerging. Limited guidance on how to safely and effectively utilize available therapies poses challenges for healthcare providers. Herein, we provide generally accepted principles, consensus recommendations, and a treatment algorithm for the management of vitiligo, as developed by a panel of ten Canadian dermatologists with expertise in managing vitiligo.
Methods: The three-phase process consisted of identifying themes and research questions; conducting a systematic literature review; and discussing/voting on generally accepted principles, consensus statements, and a treatment algorithm using an iterative consensus process.
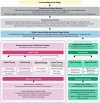
Results: Experts agreed to 27 generally accepted principles, ten consensus statements, and a treatment algorithm. Education about vitiligo pathogenesis and repigmentation biology can help patients, caregivers, and healthcare providers set realistic expectations for treatment. Treatment should focus on repigmentation or stabilizing progression, rather than on depigmentation. Topical therapies include topical corticosteroids, topical calcineurin inhibitors, and the topical Janus kinase inhibitor ruxolitinib cream. Phototherapy, such as narrow-band ultraviolet B and excimer laser/lamp, can be used as monotherapy or in combination with other treatments. Off-label systemic therapies may be appropriate for patients with unstable or rapidly progressing disease. Surgical therapy may be suitable for patients with localized or stable recalcitrant disease. Maintenance therapy may help mitigate the risk of disease relapse.
Conclusion: Improved clarity around the benefits, risks, and limitations of available therapies has supported the development of robust guidelines and a treatment algorithm for vitiligo. Disease stabilization and repigmentation are goals that can largely be achieved, particularly when patients share a mutual understanding of vitiligo and its treatment options. A Graphical Abstract is available for this article.
Keywords: Guidelines; Janus kinase inhibitor; Phototherapy; Topical calcineurin inhibitor; Topical corticosteroid; Vitiligo.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Vimal H. Prajapati has served as an advisor, consultant, and/or speaker for AbbVie, Actelion, Amgen, Apogee Therapeutics, Aralez, Arcutis, Aspen, Bausch Health, BioScript Solutions, Boehringer Ingelheim, Bristol Myers Squibb, Canadian Psoriasis Network, Celgene, Celltrion, Cipher, Concert, CorEvitas, Eczema Society of Canada, Eli Lilly, Galderma, GlaxoSmithKline, Homeocan, Incyte Corporation, JAMP Pharma, Janssen, Johnson & Johnson, LEO Pharma, Medexus, Novartis, Organon, Pediapharm, Pfizer, Sanofi Genzyme, Sun Pharma, Tribute, UCB, and Valeant; served as an investigator for AbbVie, AnaptysBio, Arcutis, Arena, Asana, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Concert, CorEvitas, Dermavant, Dermira, Eli Lilly, Galderma, Incyte Corporation, Janssen, LEO Pharma, Meiji Pharma, Nektar Therapeutics, Nimbus Lakshmi, Novartis, Pfizer, RAPT Therapeutics, Regeneron, Reistone, Sanofi Genzyme, Sun Pharma, Takeda, and UCB; and received grants from AbbVie, Bausch Health, Janssen, LEO Pharma, Novartis, and Sanofi Genzyme. Harvey Lui has served as an advisor, consultant, investigator, and/or speaker for AbbVie, Incyte Corporation, L’Oréal, Novartis, and Vita Imaging. Yvette Miller-Monthrope has served as an advisor, consultant, and/or speaker for AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Fresenius Kabi, Galderma, Incyte Corporation, Janssen, Sanofi, Sun Pharma, Novartis, and UCB. Julien Ringuet has served as an advisor, consultant, and/or speaker for AbbVie, Amgen, Apogee, Arcutis, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galderma, Incyte Corporation, Janssen, LEO Pharma, L’Oréal, NKS Health, Novartis, Organon, Pfizer, Sandoz, Sanofi Genzyme, Sun Pharma, and UCB; and served as an investigator for AbbVie, Alumis, Amgen, Apogee, Aristea, Aslan, Bristol Myers Squibb, Celgene, Concert Pharmaceuticals, Correvitas, DICE Therapeutics, Incyte Corporation, Innovaderm, Janssen, Kyowa Kirin, LEO Pharma, Merck, Pfizer, Sanofi Genzyme, Sun Pharma, and UCB. Irina Turchin has served as a speaker, advisor, consultant, or investigator for AbbVie, Amgen, Arcutis, Aristea, Bausch Health, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Galderma, Horizon Therapeutics, Incyte Corporation, Janssen, Kiniksa, LEO Pharma, Mallinckrodt, MoonLake, Novartis, Pfizer, Sanofi, Sun Pharma, UCB, and Ventyx Biosciences. H. Chih-ho Hong has served as a speaker, advisor, consultant, and/or investigator for AbbVie, Amgen, Arcutis, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cutanea, Dermira, Dermavant, DS Biopharma, Eli Lilly, Galderma, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Medimmune, Merck, Mirimar, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Roche, and UCB. Charles Lynde has served as a speaker and/or consultant for AbbVie, Amgen, Aralez, Arcutis, Bausch Health, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cipher, Dermavant, Eli Lilly, Fresnius Kabi, Galderma, GlaxoSmithKline, Incyte Corporation, Innovaderm, Intega Skin, Janssen, Kyowa Kirin, La Roche Posay, LEO Pharma, L’Oréal, Medexus, MedX, Merck, Novartis, P&G, Pediapharm, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sandoz, Sentrex, Sun Pharma, TEVA, Tribute, UCB, Valeant, Viatris, and Volo Health; and has served as a principal investigator for AbbVie, Acelyrin, Akros, Altius, Amgen, Aralez, Arcutis, Avillion, Bausch Health, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Cipher, Concert, Dermavant, Devonian, Eli Lilly, Evelo, Galderma, GlaxoSmithKline, Incyte Corporation, Innovaderm, Intega Skin, Janssen, Kyowa Kirin, La Roche Posay, LEO Pharma, L’Oréal, Medexus, MedX, Merck, MoonLake, Novartis, P&G, Pediapharm, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sandoz, Sentrex, Sun Pharma, TEVA, Tribute, UCB, Valeant, Viatris, and Volo Health. Kim A. Papp has received honoraria and/or grants as a consultant, speaker, investigator, or scientific officer from AbbVie, Acelyrin, Akros, Alumis, Amgen, Arcutis, Bausch Health/Valeant, Boehringer Ingelheim, Bristol Myers Squibb, Can-Fite Biopharma, Celltrion, Concert Pharmaceuticals, Dermavant, Dermira, DICE Pharmaceuticals, DICE Therapeutics, Eli Lilly and Company, Evelo Biosciences, Forbion, Galderma, Horizon Therapeutics, Incyte Corporation, Janssen, Kymab, Kyowa Hakko Kirin, LEO Pharma, Meiji Seika Pharma, Mitsubishi Pharma, Nimbus Therapeutics, Novartis, Pfizer, Reistone, Sanofi-Aventis/Genzyme, Sandoz, Sun Pharma, Takeda, Tarsus Pharmaceuticals, UCB Pharma, and Zai Lab. Jensen Yeung has served as a consultant, investigator, or speaker or received honoraria from AbbVie, Amgen, Acrutis, Apogee, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Fresenius Kabi, Galderma, Incyte Corporation, JAMP Pharma, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, Takeda, and UCB. Melinda J. Gooderham has served as a principal investigator for AbbVie, Alumis, Amgen, AnaptysBio, Apogee, Arcutis, Aristea, Aslan, Bausch Health, Boehringer Ingelheim International GmbH, Bristol Myers Squibb, Cara Therapeutics, Coherus Biosciences, Dermira, Eli Lilly, Galderma SA, GlaxoSmithKline, Incyte Corporation, InMagene, JAMP, Janssen, LEO Pharma, MedImmune, Meiji, MoonLake, Nektar Therapeutics, Nimbus, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, Takeda Pharmaceutical Company, Tarsus, UCB, Ventyx, and Vyne; a consultant for AbbVie, Amgen, Apogee, Aslan, Bausch Health, Boehringer Ingelheim International GmbH, Eli Lilly, Janssen, Novartis Pharmaceuticals, Sanofi Genzyme, Sun Pharma, and UCB; an advisory board member for AbbVie, Amgen, Apogee, Arena Pharmaceuticals, Asana BioSciences, Aslan, Bausch Health, Boehringer Ingelheim International GmbH, Eli Lilly, Galderma SA, Incyte Corporation, Janssen, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, UCB, and Union; and a paid speaker for AbbVie, Amgen, Bausch Health, Bristol Myers Squibb, Boehringer Ingelheim International GmbH, Eli Lilly, Galderma SA, Janssen, JAMP, LEO Pharma, L’Oréal, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, and UCB. Ethical approval: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Figures
Similar articles
-
Management of vitiligo: a promising future.Ital J Dermatol Venerol. 2025 Jun;160(3):248-261. doi: 10.23736/S2784-8671.25.08112-5. Epub 2025 May 8. Ital J Dermatol Venerol. 2025. PMID: 40338195 Review.
-
Canadian Consensus Guidelines for the Management of Atopic Dermatitis with Topical Therapies.Dermatol Ther (Heidelb). 2025 Jun;15(6):1467-1485. doi: 10.1007/s13555-025-01386-2. Epub 2025 Apr 25. Dermatol Ther (Heidelb). 2025. PMID: 40279086 Free PMC article.
-
Home-based narrowband UVB, topical corticosteroid or combination for children and adults with vitiligo: HI-Light Vitiligo three-arm RCT.Health Technol Assess. 2020 Nov;24(64):1-128. doi: 10.3310/hta24640. Health Technol Assess. 2020. PMID: 33245043 Free PMC article. Clinical Trial.
-
[Vitiligo-update on pathogenesis, diagnostics and therapy].Dermatologie (Heidelb). 2025 Mar;76(3):168-178. doi: 10.1007/s00105-024-05467-9. Epub 2025 Feb 4. Dermatologie (Heidelb). 2025. PMID: 39904778 Review. German.
-
Effect of narrow band ultraviolet B phototherapy as monotherapy or combination therapy for vitiligo: a meta-analysis.Photodermatol Photoimmunol Photomed. 2017 Jan;33(1):22-31. doi: 10.1111/phpp.12277. Epub 2016 Nov 23. Photodermatol Photoimmunol Photomed. 2017. PMID: 27696531 Review.
Cited by
-
Potential of automated image analysis for the measurement of vitiligo lesions.Front Med (Lausanne). 2025 Aug 14;12:1623408. doi: 10.3389/fmed.2025.1623408. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40893879 Free PMC article. No abstract available.
References
-
- Sandoval-Cruz M, Garcia-Carrasco M, Sanchez-Porras R, Mendoza-Pinto C, Jimenez-Hernandez M, Munguia-Realpozo P, et al. Immunopathogenesis of vitiligo. Autoimmun Rev. 2011;10(12):762–5. - PubMed
-
- Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatology. 2020;236(6):571–92. - PubMed
-
- Thompson AR, Clarke SA, Newell RJ, Gawkrodger DJ, Appearance Research Collaboration. Vitiligo linked to stigmatization in British South Asian women: a qualitative study of the experiences of living with vitiligo. Br J Dermatol. 2010;163(3):481–6. - PubMed
LinkOut - more resources
Full Text Sources

