GPX4 deficiency-induced ferroptosis drives endometrial epithelial fibrosis in polycystic ovary syndrome
- PMID: 40253746
- PMCID: PMC12023900
- DOI: 10.1016/j.redox.2025.103615
GPX4 deficiency-induced ferroptosis drives endometrial epithelial fibrosis in polycystic ovary syndrome
Abstract
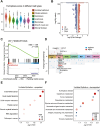
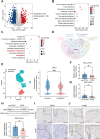
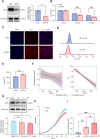
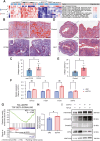
The increased risk of infertility and endometrial lesions (such as endometrial hyperplasia or cancer) in polycystic ovary syndrome (PCOS) are closely associated with the lack of cyclical transformation in the endometrium. However, the underlying mechanisms remain incompletely understood. Though integrating single-cell RNA-sequencing, transcriptomics, and metabolomics analysis, we found that glutathione (GSH) metabolism disorder and the overactivation of ferroptosis, triggered by glutathione peroxidase 4 (GPX4) deficiency in endometrial epithelial cells, were the consequences of the prolonged endometrial proliferative phase in PCOS. This change may collectively contribute to some extent to decidualization failure. We further performed GSVA analysis and determined that the negative correlation between ferroptosis and fibrosis-related pathway was the most significant. Therefore, we first confirmed the presence of fibrosis in the proliferative endometrium of PCOS and PCOS-like mouse uteri. Additionally, by establishing endometrial organoids (EEOs) models and in vitro cell line models, we demonstrated that GPX4 deficiency contributed to extracellular matrix remodeling and excessive collagen deposition, via activating the TGF-β1/Smad2/3 pathway, which ultimately accelerated fibrosis. GSH intervention to the EEOs of PCOS could alleviate their fibrotic phenotypes at different stages. These findings may serve as a promising therapeutic target for PCOS-related endometrial dysfunction, as well as valuable strategies for improving PCOS-related adverse pregnancy outcomes.
Keywords: Endometrial fibrosis; Female infertility; Ferroptosis; Organoid; Polycystic ovary syndrome.
Copyright © 2025. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

