Efficacy and safety of mesenchymal stem cell therapy in acute on chronic liver failure: a systematic review and meta-analysis of randomized controlled clinical trials
- PMID: 40254564
- PMCID: PMC12010635
- DOI: 10.1186/s13287-025-04303-8
Efficacy and safety of mesenchymal stem cell therapy in acute on chronic liver failure: a systematic review and meta-analysis of randomized controlled clinical trials
Abstract
Background: Acute-on-chronic liver failure has become a serious global health burden, which is characterized by an acute deterioration of liver function, rapidly evolving organ failure, and high short-term mortality in patients with chronic liver disease. The pathogenesis includes extensive hepatic necrosis, which is related to intense systemic inflammation and subsequently causes the inflammatory cytokine storm, resulting in portal hypertension, organ dysfunction, and organ failure. Mesenchymal stem cells can function as seed cells to remodel and repair damaged liver tissues, thus showing potential therapeutic alternatives for patients with chronic liver disease. However, standard treatment protocols for mesenchymal stem cells in acute-on-chronic liver failure patients have not been established.
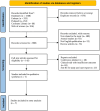
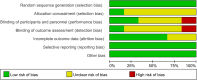
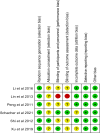
Methods: We conducted a detailed search from PubMed/Medline, Web of Science, EMBASE, and Cochrane Library to find randomized controlled trials published before October 23, 2021. We formulated criteria for the literature screening according to the PICOS principle (Population, Intervention, Comparison, Outcome, Study design). Subsequently, the bias risk assessment tool was used to assess the quality of all enrolled studies. Finally, outcome measurements including the model of end-stage liver disease score, albumin, total bilirubin, coagulation function, and aminotransferase were extracted for statistical analysis.
Results: A total of 7 clinical trials were included. The results of enrolled studies indicated that patients with acute-on-chronic liver failure who received mesenchymal stem cells inoculation showed a decreased MELD score in 4 weeks and 24 weeks, compared with counterparts who received conventional treatment. Reciprocally, mesenchymal stem cells inoculation improved the ALB levels in 4 weeks and 24 weeks. For secondary indicators, mesenchymal stem cells treatment significantly reduced INR levels and ALT levels, compared with the control group. Our results showed no significant differences in the incidence of adverse reactions or serious adverse events monitored in patients after mesenchymal stem cells inoculation.
Conclusion: This meta-analysis indicated that mesenchymal stem cell infusion is effective and safe in the treatment of patients with acute-on-chronic liver failure. Without increasing the incidence of adverse events or serious adverse events, MSC treatment improved liver function including a decrease in MELD score and an increase in ALB levels in patients with acute-on-chronic liver failure. However, large-cohort randomized controlled trials with longer follow-up periods are required to further confirm our conclusions.
Keywords: Acute-on-chronic liver failure; Efficacy; Mesenchymal stem cells; Meta-analysis; Safety.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Conflict of interest: The authors declare that they have no conflict of interest.
Figures
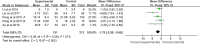



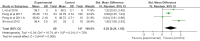
Similar articles
-
Efficacy and safety of mesenchymal stem cells therapy in COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials.J Transl Med. 2024 Jun 8;22(1):550. doi: 10.1186/s12967-024-05358-6. J Transl Med. 2024. PMID: 38851730 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Granulocyte colony-stimulating factor with or without stem or progenitor cell or growth factors infusion for people with compensated or decompensated advanced chronic liver disease.Cochrane Database Syst Rev. 2023 Jun 6;6(6):CD013532. doi: 10.1002/14651858.CD013532.pub2. Cochrane Database Syst Rev. 2023. PMID: 37278488 Free PMC article.
References
-
- Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–37, 1437.e1421-1429. - PubMed
-
- Artru F, McPhail MJ. Immunopathogenesis of acute on chronic liver failure. Am J Transpl. 2024;24(5):724–32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

