Neutrophil infiltration and microglial shifts in sepsis induced preterm brain injury: pathological insights
- PMID: 40254577
- PMCID: PMC12010587
- DOI: 10.1186/s40478-025-02002-2
Neutrophil infiltration and microglial shifts in sepsis induced preterm brain injury: pathological insights
Abstract
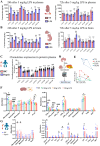
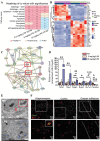
Preterm sepsis is a major contributor to brain injury and long-term neurodevelopmental impairments, but its molecular mechanisms remain poorly understood. This study integrated clinical and experimental approaches to investigate the pathological changes linking systemic inflammation to brain injury in preterm infants. Transcriptomic analysis of septic preterm infants' peripheral blood revealed upregulated immune, metabolic, and inflammatory pathways, suggesting a link between systemic and brain inflammation. Using P2 mice, we established a preterm white matter injury model through multiple doses of lipopolysaccharide, observing dose-dependent developmental delays, brain inflammation, and long-term behavioral deficits. Integrative analyses of peripheral blood and brain samples from both mice and preterm infants revealed consistent chemokine alterations and immune cell infiltration across peripheral and central compartments, highlighting the significant involvement of neutrophil extracellular traps in preterm brain injury. Furthermore, microglia exhibited significant transcriptional changes during the acute phase, accompanied by metabolic reprogramming from oxidative phosphorylation to glycolysis, with suggested involvement of Pgk1 and Pgam1. This shift intensified with escalating inflammation, along with PANoptosis-related gene upregulation, ultimately associated with microglial cell death. Collectively, these findings provide pathological insights into the immunometabolic alterations underlying sepsis-induced preterm brain injury and suggest potential targets for future therapeutic interventions to mitigate long-term neurodevelopmental deficits.
Keywords: Behavioral deficits; Microglial metabolic reprogramming; Neonatal sepsis; Neutrophil; PANoptosis; White matter injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Ethical approval for the preterm infants study was obtained from the Ethics Committees of The Third Affiliated Hospital of Zhengzhou University (ethical no: 2018-04). All procedures for the animal study followed the institutional animal care standards set by the Ethics Committee of Zhengzhou University [Ethics number 2022-(yu)121]. Competing interests: The authors declare no competing interests.
Figures








Similar articles
-
Safety and feasibility of allogeneic cord blood-derived cell therapy in preterm infants with severe brain injury (ALLO trial): a phase-1 trial protocol.BMJ Open. 2025 Jun 18;15(6):e100389. doi: 10.1136/bmjopen-2025-100389. BMJ Open. 2025. PMID: 40533219 Free PMC article.
-
Different corticosteroids and regimens for accelerating fetal lung maturation for babies at risk of preterm birth.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD006764. doi: 10.1002/14651858.CD006764.pub4. Cochrane Database Syst Rev. 2022. PMID: 35943347 Free PMC article.
-
Late (> 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants.Cochrane Database Syst Rev. 2017 Oct 24;10(10):CD001145. doi: 10.1002/14651858.CD001145.pub4. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Nov 11;11:CD001145. doi: 10.1002/14651858.CD001145.pub5. PMID: 29063594 Free PMC article. Updated.
-
Late (>7 days) postnatal corticosteroids for chronic lung disease in preterm infants.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD001145. doi: 10.1002/14651858.CD001145.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2014 May 13;(5):CD001145. doi: 10.1002/14651858.CD001145.pub3. PMID: 19160189 Updated.
-
Persistent inflammation and white matter damage in the preterm brain: Insights from a novel ovine model of chronic inflammation.Exp Neurol. 2025 Nov;393:115397. doi: 10.1016/j.expneurol.2025.115397. Epub 2025 Jul 24. Exp Neurol. 2025. PMID: 40714018
References
-
- Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M et al (2010) Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40:1423–1430. 10.1007/s10803-010-1006-y - PubMed
-
- Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI et al (2019) A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab 30:493–507e496. 10.1016/j.cmet.2019.06.005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

