Cervical cancer screening: a comparative study of TruScreen vs. Pap Smear
- PMID: 40254583
- PMCID: PMC12010667
- DOI: 10.1186/s12905-025-03733-z
Cervical cancer screening: a comparative study of TruScreen vs. Pap Smear
Abstract
Objectives: This study aimed to evaluate the potential of real-time optoelectronic device (TruScreen™; TS; TruScreen Group Limited, New Zealand) as an alternative or adjunct to Pap Smear (Liquid Based Cytology (LBC)) for cervical cancer screening.
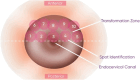
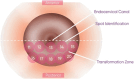
Method: We conducted a prospective observational pilot study involving 507 women who were routinely followed at gynecology clinics. All participants underwent TS and LBC examinations after study enrolment. Those with abnormal findings were referred for colposcopy and cervical biopsy within one month.
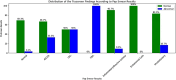
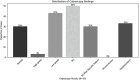
Results: Overall, 507 women fulfilled the eligibility criteria and were included in this study, of which 30 women (5.9%) had abnormal TS findings and underwent colposcopy. Thirteen women (43.3%) had low-grade lesions, and only one (3.3%) had a high-grade lesion. Regarding biopsy findings, three women had cervical intraepithelial neoplasia (CIN) 1, two women had 'CIN2 + , and one had glandular hyperplasia. The TS yielded a sensitivity of 83.3% (95% CI: 35.9-99.6%) and a specificity of 95% (95% CI: 92.7- 96.8%) for the detection of cervical abnormality, compared to 66.7% (95% CI: 22.3-95.7%) and 98.2% (95%: CI 96.6%-99.2%) of the Pap smear, respectively. The difference between both screening tools was not statistically significant (p = 0.91). The sensitivity (100%, 95% CI 15.6-100%) and specificity (95.6%, 95% CI 93.4-97.2%) of TS and Pap smear for 'CIN2 + lesions were notably high.
Conclusion: TS demonstrated potential as a screening tool for cervical neoplasms in this preliminary study. The tool did not require cervical samples, laboratory equipment, or highly trained personnel. While our findings suggest the potential for real-time and accurate screening, further research with a larger sample size is necessary to confirm its reliability and practicality.
Keywords: CIN; Cervical cancer; Optoelectronic cervical screening; Optoelectronic device; Pap smear; Real-time screening; TruScreen.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the institutional review board (IRB) (Ref No. Dr. Sulaiman Al-Habib Medical Group – RC20.08.91- Aug 2020). All patients were required to sign the informed consent before enrolment. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
A real time optoelectronic device as an adjunct to the Pap smear for cervical screening: a multicenter evaluation.Int J Gynecol Cancer. 2003 Nov-Dec;13(6):804-11. doi: 10.1111/j.1525-1438.2003.13393.x. Int J Gynecol Cancer. 2003. PMID: 14675317 Clinical Trial.
-
Comparison of the detection rate of cervical lesion with TruScreen, LBC test and HPV test: A Real-world study based on population screening of cervical cancer in rural areas of China.PLoS One. 2020 Jul 7;15(7):e0233986. doi: 10.1371/journal.pone.0233986. eCollection 2020. PLoS One. 2020. PMID: 32634143 Free PMC article.
-
Evaluation of a real-time optoelectronic method in the diagnostics of CIN over four years of observations.PLoS One. 2021 Feb 26;16(2):e0247702. doi: 10.1371/journal.pone.0247702. eCollection 2021. PLoS One. 2021. PMID: 33635909 Free PMC article.
-
Screening test accuracy of portable devices that can be used to perform colposcopy for detecting CIN2+ in low- and middle-income countries: a systematic review and meta-analysis.BMC Womens Health. 2020 Nov 16;20(1):253. doi: 10.1186/s12905-020-01121-3. BMC Womens Health. 2020. PMID: 33198721 Free PMC article.
-
Colposcopy in the Primary Health Care: A Scoping Review.J Prim Care Community Health. 2023 Jan-Dec;14:21501319231198942. doi: 10.1177/21501319231198942. J Prim Care Community Health. 2023. PMID: 37740513 Free PMC article.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J cancer. 2015;136(5):E359–86. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. - PubMed
-
- Mandelblatt JS, Lawrence WF, Gaffikin L, Limpahayom KK, Lumbiganon P, Warakamin S, et al. Costs and Benefits of Different Strategies to Screen for Cervical Cancer in Less-Developed Countries. JNCI J Natl Cancer Inst. 2002;94(19):1469–83. - PubMed
-
- Allameh T, Khanjani S, Mohammadizadeh F, Refaei E. Diagnostic value of the combination of TruScreen and Pap smear in screening cervical epithelial lesions: Does it add advantages over the Pap smear alone? Open J Obstet Gynecol. 2013;03(03):341–6.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

