Neural-induced human adipose tissue-derived stem cell secretome exerts neuroprotection against rotenone-induced Parkinson's disease in rats
- PMID: 40254594
- PMCID: PMC12010609
- DOI: 10.1186/s13287-025-04306-5
Neural-induced human adipose tissue-derived stem cell secretome exerts neuroprotection against rotenone-induced Parkinson's disease in rats
Abstract
Background: Parkinson's disease (PD) is a multifactorial disease that involves genetic and environmental factors, which play an essential role in the pathogenesis of PD. Mesenchymal stem cells release a set of bioactive molecules called "secretome" that regulates intercellular communication and cargo transfer in signaling pathways for PD treatment. Thus, this study aimed to evaluate the neuroprotective effects of neural-induced human adipose tissue-derived stem cell (NI-hADSC)-conditioned medium (NI-hADSC-CM) and its exosomes (NI-hADSC-Exo) in a rotenone (ROT)-induced model of PD in rats.
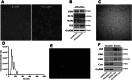
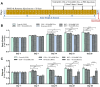
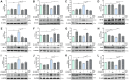
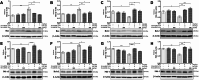
Methods: The NI-hADSC-CM was collected from NI-hADSC after 14 days of neural differentiation, and its NI-hADSC-Exo were isolated using a tangential flow filtration system. ROT (1 mg/kg) was subcutaneously administered for 28 days to establish a model of PD in rats. The treatment of NI-hADSC-CM or NI-hADSC-Exo was intravenously injected on days 15, 18, 21, 24, and 27. Animal behavioral effects were explored via a rotarod test. After 28 days, histological and western blot analyses were performed to investigate the tyrosine hydroxylase (TH), α-synuclein (α-syn) aggregation, and downstream signaling pathways for experimental validation.
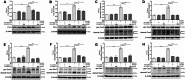
Results: NI-hADSC-Exo improved the motor balance and coordination skills against ROT toxicity. ROT reproduced the pathological features of PD, such as a decrease in TH-positive dopaminergic neurons and an increase in α-syn aggregation and glial fibrillary acidic protein (GFAP)-positive cells. NI-hADSC-CM and NI-hADSC-Exo improved the TH expression, decreased the Triton X-100 soluble and insoluble oligomeric p-S129 α-syn, and influenced the differential reactivity to astrocytes and microglia. Secretome treatment could reverse the ROT-induced damages in the neuronal structural and functional proteins, mitochondrial apoptosis, and caspase cascade. The treatment of NI-hADSC-CM and NI-hADSC-Exo ameliorated the ROT toxicity-induced serine-threonine protein kinase dysregulation and autophagy impairment to clear the aggregated α-syn.
Conclusions: NI-hADSC-CM and NI-hADSC-Exo significantly exerted neuroprotection by decreasing α-syn toxicity, inhibiting neuroinflammation and apoptosis, restoring autophagic flux properties, and promoting the neuronal function in ROT-injected rats; however, the influence of these treatments on signaling pathways differed slightly between the midbrain and striatum regions. Targeting α-syn degradation pathways provides a novel strategy to elucidate the beneficial effects of MSC secretome and future safe cell-free treatments for PD.
Keywords: Alpha-synuclein; Autophagy; Exosomes; Neuronal markers; Protein kinases.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: Adipose tissues from human donors were collected in accordance with the guidelines approved by the Chonnam National University Hospital Biomedical Research Ethics Review Committee and that informed consent was obtained from the donors (title: Establishment of cell resources and specific therapeutic techniques for nervous system diseases using human mesenchymal stem cells, committee: Chonnam National University Hospital Institutional Review Board, approval number: CNUH IRB-I-2009-03-016, date of approval: 25 March 2009, expiration date: 2 September 2025). All animal experimental procedures were conducted in accordance with the ARRIVE guidelines and approved by the Animal Experiment Ethics Committee of the Chonnam National University Medical and Life Science Convergence Center (title: Stem Cell-derived New Drug Development, committee: Chonnam National University Institutional Animal Care and Use Committee, approval number: CNU IACUC-H-2022-36, date of approval: 4 May 2022, period: 8 May 2022 ~ 7 May 2023; title: Stem Cell-derived New Drug Development, committee: Chonnam National University Institutional Animal Care and Use Committee, approval number: CNU IACUC-H-2023-11, date of approval: 19 April 2023, period: 20 April 2023 ~ 19 April 2024). Consent for publication: Not applicable. Competing interests: The authors declare no competing interest.
Figures







References
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in lewy bodies. Nature. 1997;388:839–40. - PubMed
-
- Mehra S, Sahay S, Maji SK. alpha-Synuclein misfolding and aggregation: implications in Parkinson’s disease pathogenesis. Biochim Biophys Acta Proteins Proteom. 2019;1867:890–908. - PubMed
-
- Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H. Overexpression of alpha-synuclein in rat substantia Nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson’s disease. J Neurochem. 2004;91:451–61. - PubMed
MeSH terms
Substances
Grants and funding
- NRF-2020R1I1A3070388/National Research Foundation of Korea
- NRF-2020R1F1A1076616/National Research Foundation of Korea
- NRF-2021R1I1A3060435/National Research Foundation of Korea
- BCRI23041/Chonnam National University Hospital Biomedical Research Institute
- BCRI25048/Chonnam National University Hospital Biomedical Research Institute
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

