Role of gut microbiome in suppression of cancers
- PMID: 40254597
- PMCID: PMC12013426
- DOI: 10.1080/19490976.2025.2495183
Role of gut microbiome in suppression of cancers
Abstract
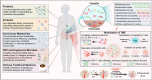
The pathogenesis of cancer is closely related to the disruption of homeostasis in the human body. The gut microbiome plays crucial roles in maintaining the homeostasis of its host throughout lifespan. In recent years, a large number of studies have shown that dysbiosis of the gut microbiome is involved in the entire process of cancer initiation, development, and prognosis by influencing the host immune system and metabolism. Some specific intestinal bacteria promote the occurrence and development of cancers under certain conditions. Conversely, some other specific intestinal bacteria suppress the oncogenesis and progression of cancers, including inhibiting the occurrence of cancers, delaying the progression of cancers and boosting the therapeutic effect on cancers. The promoting effects of the gut microbiome on cancers have been comprehensively discussed in the previous review. This article will review the latest advances in the roles and mechanisms of gut microbiome in cancer suppression, providing a new perspective for developing strategies of cancer prevention and treatment.
Keywords: Gut microbiome; cancer; suppression.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
The Microbiome Matters: Its Impact on Cancer Development and Therapeutic Responses.J Microbiol. 2024 Mar;62(3):137-152. doi: 10.1007/s12275-024-00110-7. Epub 2024 Apr 8. J Microbiol. 2024. PMID: 38587593 Review.
-
Gut microbiota in cancer initiation, development and therapy.Sci China Life Sci. 2025 May;68(5):1283-1308. doi: 10.1007/s11427-024-2831-x. Epub 2024 Dec 30. Sci China Life Sci. 2025. PMID: 39821827 Review.
-
Exploring the gut microbiome: A potential biomarker for cancer diagnosis, prognosis, and therapy.Biochim Biophys Acta Rev Cancer. 2025 Feb;1880(1):189251. doi: 10.1016/j.bbcan.2024.189251. Epub 2024 Dec 22. Biochim Biophys Acta Rev Cancer. 2025. PMID: 39719176 Review.
-
Gut microbiome in multiple sclerosis: The players involved and the roles they play.Gut Microbes. 2017 Nov 2;8(6):607-615. doi: 10.1080/19490976.2017.1349041. Epub 2017 Aug 3. Gut Microbes. 2017. PMID: 28696139 Free PMC article. Review.
-
Dysbiosis of the Gut Microbiome is associated with Tumor Biomarkers in Lung Cancer.Int J Biol Sci. 2019 Sep 7;15(11):2381-2392. doi: 10.7150/ijbs.35980. eCollection 2019. Int J Biol Sci. 2019. PMID: 31595156 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
