PI3K-Akt signalling regulates Scx-lineage tenocytes and Tppp3-lineage paratenon sheath cells in neonatal tendon regeneration
- PMID: 40254618
- PMCID: PMC12010001
- DOI: 10.1038/s41467-025-59010-y
PI3K-Akt signalling regulates Scx-lineage tenocytes and Tppp3-lineage paratenon sheath cells in neonatal tendon regeneration
Abstract
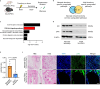
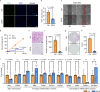
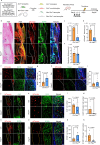
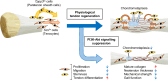
Tendon injuries are frequently occurring disorders; it is clinically important to enhance tendon regeneration and prevent functional impairment post-injury. While tendon injuries in children heal quickly with minimal scarring, those in adults heal slowly and are accompanied by fibrotic scarring. Therefore, investigating the healing mechanisms after tendon injury, and identifying the factors that regulate the inherent regenerative capacity of tendons are promising approaches to promoting tendon regeneration. Here, we identify that the PI3K-Akt signalling pathway is preferentially upregulated in injured neonatal murine Achilles tendons. Inhibition of PI3K-Akt signalling in a neonatal murine Achilles tendon rupture model decreases cell proliferation and migration in both Scx-lineage intrinsic tenocytes and Tppp3-lineage extrinsic paratenon sheath cells. Moreover, the inhibition of PI3K-Akt signalling decreases stemness and promotes mature tenogenic differentiation in both Scx- and Tppp3-lineage cells. Collectively, these results suggest that PI3K-Akt signalling plays a pivotal role in neonatal tendon regeneration.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Voleti, P. B., Buckley, M. R. & Soslowsky, L. J. Tendon healing: repair and regeneration. Annu. Rev. Biomed. Eng.14, 47–71 (2012). - PubMed
-
- Hart, D., Kydd, A. & Reno, C. Gender and pregnancy affect neuropeptide responses of the rabbit Achilles tendon. Clin. Orthop. Relat. Res.365, 237–246 (1999). - PubMed
-
- Shaw, A. V. et al. Outcome measurement in adult flexor tendon injury: a systematic review. J. Plast. Reconstr. Aesthet. Surg.75, 1455–1466 (2022). - PubMed
MeSH terms
Substances
Grants and funding
- 18K09101/MEXT | Japan Society for the Promotion of Science (JSPS)
- 18K16617/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21H03052/MEXT | Japan Society for the Promotion of Science (JSPS)
- 23K21465/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP18K09101/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

