CITK modulates BRCA1 recruitment at DNA double strand breaks sites through HDAC6
- PMID: 40254670
- PMCID: PMC12009987
- DOI: 10.1038/s41419-025-07655-4
CITK modulates BRCA1 recruitment at DNA double strand breaks sites through HDAC6
Abstract
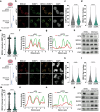
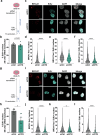
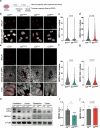
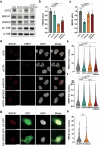
Citron Kinase (CITK) is a protein encoded by the CIT gene, whose pathogenic variants underlie microcephalic phenotypes that characterize MCPH17 syndrome. In neural progenitors, CITK loss leads to microtubule instability, resulting in mitotic spindle positioning defects, cytokinesis failure, and accumulation of DNA double strand breaks (DSBs), ultimately resulting in TP53-dependent senescence and apoptosis. Although DNA damage accumulation has been associated with impaired homologous recombination (HR), the role of CITK in this process and whether microtubule dynamics are involved is still unknown. In this report we show that CITK is required for proper BRCA1 localization at sites of DNA DSBs. We found that CITK's scaffolding, rather than its catalytic activity, is necessary for maintaining BRCA1 interphase levels in progenitor cells during neurodevelopment. CITK regulates the nuclear levels of HDAC6, a modulator of both microtubule stability and DNA damage repair. Targeting HDAC6 in CITK-deficient cells increases microtubule stability and recovers BRCA1 localization defects and DNA damage levels to that detected in controls. In addition, the CIT-HDAC6 axis is functionally relevant in a MCPH17 zebrafish model, as HDAC6 targeting recovers the head size phenotype produced by interfering with the CIT orthologue gene. These data provide novel insights into the functional interplay between HR and microtubule dynamics and into the pathogenesis of CITK based MCPH17, which may be relevant for development of therapeutic strategies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: For iPSC lines, subjects were enrolled according to protocols approved by institutional review boards at affiliated institutions, as previously described [11]. In all cases, the procedures followed were in accordance with the ethical standards of the respective institution’s committee on human research and were in keeping with international standards. Written informed consent was obtained prior to participation. The mouse studies were designed according to the guidelines of the NIH, the European Communities Council (2010/63/EU) and the Italian Law for Care and Use of Experimental Animals (DL26/2014), under permission number 1128/2020-PR, released on 16th November 2020 from Italian Ministry of Health, Department of Public Veterinary Health. It was also approved by the Italian Ministry of Health and the Bioethical Committee of the University of Turin. Zebrafish were bred following international (EU Directive 2010/63/EU; European recommendations) and national recognized guidelines (Italian decree 4th March 2014, n. 26), which focus on the protection of animals used for scientific research.
Figures

References
MeSH terms
Substances
Grants and funding
- IG23341/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- PhD fellowship/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- Post doctoral fellowship/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- IG29187/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- IG29187/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- PRIN-PNRR program n. 2022M75NN8/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
- PhD fellowship/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
- INFRAP Locneuro/Regione Piemonte (Region of Piedmont)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

