A medium-chain fatty acid analogue prevents endotoxin liver injury in a murine model
- PMID: 40254678
- PMCID: PMC12009971
- DOI: 10.1038/s41598-025-98200-y
A medium-chain fatty acid analogue prevents endotoxin liver injury in a murine model
Abstract
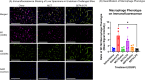
Parenteral nutrition (PN) is lifesaving for patients with short bowel syndrome and other gastrointestinal disorders, however long-term use may lead to complications including hepatosteatosis and sepsis. We have previously demonstrated the anti-steatotic, -fibrotic, and -inflammatory properties of SEFA-6179, an engineered medium-chain fatty acid analogue. We hypothesized that SEFA-6179 treatment would protect against endotoxin-induced liver injury in a murine model of PN-induced hepatosteatosis. C57Bl/6J mice were administered a high-carbohydrate liquid diet plus intravenous lipid emulsion (Intralipid, 4 g fat/kg/d) or intravenous saline for 19 days to induce hepatosteatosis. SEFA-6179 (100 mg/kg) or vehicle (MCT/medium-chain triglyceride) was administered via oral gavage for four days leading up to intraperitoneal challenge with lipopolysaccharide (15 mg/kg) or saline on day 19. Age-matched, chow-fed controls received the same treatments. The primary outcome was liver biomarkers: alanine aminotransferase and aspartate aminotransferase. Pro-inflammatory cytokines, IL-6, TNF-alpha, and monocyte chemoattractant protein (MCP1), were analyzed. Liver immunofluorescence staining was performed to evaluate macrophage phenotypes. In endotoxin-challenged mice, pre-treatment with SEFA-6179 lowered liver enzymes and pro-inflammatory cytokine levels compared to vehicle. On liver histology, SEFA-6179 pre-treatment led to greater polarization of M1/pro-inflammatory macrophages to an M2/anti-inflammatory phenotype compared to vehicle. SEFA-6179 is currently in Phase II clinical trials. These findings support the potential application of SEFA-6179 in high-risk, PN-dependent patients.
Keywords: Fatty acid; Inflammation; Liver steatosis; Parenteral nutrition.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: This investigation was performed under a sponsored research agreement and partially funded by NorthSea Therapeutics, to which MP and KG provided external consultation. A patent application has been filed for SEFA-6179 (MP). Additional funding was received from the National Institutes of Health grants 5T32HL007734 (TIH), 2T32DK007754-22 (STT), the Boston Children’s Hospital Vascular Biology Program, Boston Children’s Hospital Surgical Foundation, the Hannah Lillie Fund, the Maisie Ellis and Friends Fund, and the Luke Raymond Celaya Research Fund.
Figures





Similar articles
-
A medium-chain fatty acid analogue prevents hepatosteatosis and decreases inflammatory lipid metabolites in a murine model of parenteral nutrition-induced hepatosteatosis.PLoS One. 2023 Dec 1;18(12):e0295244. doi: 10.1371/journal.pone.0295244. eCollection 2023. PLoS One. 2023. PMID: 38039287 Free PMC article.
-
Fish oil-based injectable lipid emulsions containing medium-chain triglycerides or added α-tocopherol offer anti-inflammatory benefits in a murine model of parenteral nutrition-induced liver injury.Am J Clin Nutr. 2019 Apr 1;109(4):1038-1050. doi: 10.1093/ajcn/nqy370. Am J Clin Nutr. 2019. PMID: 30882140 Free PMC article.
-
Lipid emulsion administered intravenously or orally attenuates triglyceride accumulation and expression of inflammatory markers in the liver of nonobese mice fed parenteral nutrition formula.J Nutr. 2013 Mar;143(3):253-9. doi: 10.3945/jn.112.169797. Epub 2013 Jan 16. J Nutr. 2013. PMID: 23325918 Free PMC article.
-
A Medium-Chain Fatty Acid Analogue Prevents Intestinal Failure-Associated Liver Disease in Preterm Yorkshire Piglets.Gastroenterology. 2023 Sep;165(3):733-745.e9. doi: 10.1053/j.gastro.2023.05.035. Epub 2023 May 30. Gastroenterology. 2023. PMID: 37263310 Free PMC article.
-
The addition of medium-chain triglycerides to a purified fish oil-based diet alters inflammatory profiles in mice.Metabolism. 2015 Feb;64(2):274-82. doi: 10.1016/j.metabol.2014.10.005. Epub 2014 Oct 13. Metabolism. 2015. PMID: 25458829 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

