In Vitro and In Vivo Assessment of an Innovative Peeling System with Azelaic and Tranexamic Acids for Targeted Hyperpigmentation Reduction
- PMID: 40254690
- PMCID: PMC12033157
- DOI: 10.1007/s13555-025-01399-x
In Vitro and In Vivo Assessment of an Innovative Peeling System with Azelaic and Tranexamic Acids for Targeted Hyperpigmentation Reduction
Abstract
Introduction: Melanin, derived from tyrosine, plays a pivotal role in skin pigmentation through melanogenesis. Disruptions in this process lead to hyperpigmentation, a condition affecting skin tone and quality of life. Current treatments, including chemical peels, have limitations, highlighting the need for novel solutions. Here, we present an innovative peeling system, comprising a masque and moisturizer, formulated with a novel blend of acids, including azelaic acid (AZA) and tranexamic acid (TXA), alongside known brightening and penetration-enhancing agents for a comprehensive solution to target hyperpigmentation.
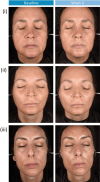
Methods: In vitro studies assessed the ability of the novel moisturizer to inhibit ultraviolet-A (UVA)-induced melanin accumulation in human melanocytes. In a single-center, controlled study, we assessed the efficacy of the peeling system in 33 healthy female participants aged 30-55 years with moderate-to-severe hyperpigmentation over a 6-week treatment period. Skin condition was assessed using clinical photography, 3D skin topography, and clinical expert evaluation (CEE) at baseline and 6 weeks post-treatment. Participants completed a self-evaluation questionnaire at 6 weeks post-treatment.
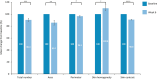
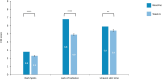
Results: In vitro findings demonstrated a concentration-dependent inhibition of melanin accumulation by the novel moisturizer. In vivo, significant reductions in dark spot number, area, and perimeter were observed at week 6, along with improvements in skin homogeneity, contrast, and brightness. Skin tone and roughness parameters also improved significantly from baseline. These findings were supported by self-evaluation findings and improvements in CEE parameters.
Conclusion: These data provide evidence for the efficacy of the innovative peeling system in reducing the appearance of hyperpigmentation over a 6-week treatment regimen in females with healthy skin and moderate-to-severe hyperpigmentation. The inclusion of AZA and TXA within the peeling system, along with active brightening and penetration-enhancing ingredients, may have synergistically facilitated the observed improvements. This multifaceted approach may address hyperpigmentation at the source, contributing to overall improvements in the appearance of the skin.
Keywords: Azelaic acid; Hyperpigmentation; Melanogenesis; Tranexamic acid.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Russell Wong has received honoraria from Dermalogica, LLC. Mariangela G. de O. Sichmann and Kevin D. Hermanson are employees of Unilever Research & Development. James Sun, Alexis R. Kim, Robert J. Bianchini, and Louis Chabert are employees of Dermalogica, LLC. Ethical Approval: Ethical approval was granted by Dermaclaim Lab S.L., Spain. The study adhered to the principles of Good Clinical Practice and the Declaration of Helsinki and its subsequent amendments. Prior to enrollment in the study, written informed consent was obtained from all participants’ for the participation and for the publication and use of all participants’ images.
Figures





Similar articles
-
Efficacy and tolerability of a depigmenting gel serum comprising tranexamic acid, niacinamide, 4-butylresorcinol, phytic acid, and a mixture of hydroxy acids that targets the biological processes regulating skin melanogenesis.J Cosmet Dermatol. 2024 Jun;23(6):2058-2065. doi: 10.1111/jocd.16148. Epub 2024 Mar 28. J Cosmet Dermatol. 2024. PMID: 38549196
-
Combination of In-Office Chemical Peels With a Topical Comprehensive Pigmentation Control Product in Skin of Color Subjects With Facial Hyperpigmentation.J Drugs Dermatol. 2017 Apr 1;16(4):301-306. J Drugs Dermatol. 2017. PMID: 28403262 Clinical Trial.
-
Hydroquinone-free Skin Brightener System for the Treatment of Moderate-to-severe Facial Hyperpigmentation.J Clin Aesthet Dermatol. 2014 May;7(5):27-31. J Clin Aesthet Dermatol. 2014. PMID: 24847406 Free PMC article.
-
Chemical peeling in ethnic/dark skin.Dermatol Ther. 2004;17(2):196-205. doi: 10.1111/j.1396-0296.2004.04020.x. Dermatol Ther. 2004. PMID: 15113287 Review.
-
Management of hyperpigmentation: Current treatments and emerging therapies.Pigment Cell Melanoma Res. 2021 Nov;34(6):1000-1014. doi: 10.1111/pcmr.12986. Epub 2021 Jun 3. Pigment Cell Melanoma Res. 2021. PMID: 33998768 Review.
References
LinkOut - more resources
Full Text Sources

