Fusogenic Nanoreactor-Based Detection of Extracellular Vesicle-derived miRNAs for Diagnosing Atherosclerosis
- PMID: 40254988
- PMCID: PMC12160682
- DOI: 10.1002/smll.202501789
Fusogenic Nanoreactor-Based Detection of Extracellular Vesicle-derived miRNAs for Diagnosing Atherosclerosis
Abstract
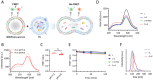
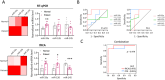
Extracellular vesicle (EV) microRNAs (miRNAs) are critical liquid-biopsy biomarkers that facilitate noninvasive clinical diagnosis and disease monitoring. However, conventional methods for detecting these miRNAs require EV lysis, which is expensive, labor-intensive, and time-consuming. Inspired by natural viral infection mechanisms, a novel strategy is developed for detecting EV miRNAs in situ via vesicle fusion mediated by viral fusion proteins. A padlock probe encapsulated within fusogenic liposomes is activated by target miRNAs, thereby initiating a highly sensitive and specific rolling circle amplification (RCA) reaction. Three EV miRNAs associated with atherosclerosis are successfully analyzed using this method, thereby enabling clear differentiation of healthy and diseased mice at several disease stages. Overall, the developed platform offers a simple approach for detecting EV miRNAs and demonstrates significant potential for broad use in applications involving disease diagnosis and monitoring.
Keywords: atherosclerosis diagnosis; extracellular vesicle; fusogenic nanoreactor; microrna; rolling circle amplification.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Single-step RT-qPCR for detection of extracellular vesicle microRNAs in vivo: a time- and cost-effective method.Am J Physiol Lung Cell Mol Physiol. 2020 Apr 1;318(4):L742-L749. doi: 10.1152/ajplung.00430.2019. Epub 2020 Feb 19. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32073880 Free PMC article.
-
Quantitative and Multiplex Detection of Extracellular Vesicle-Derived MicroRNA via Rolling Circle Amplification within Encoded Hydrogel Microparticles.Adv Healthc Mater. 2022 May;11(10):e2102332. doi: 10.1002/adhm.202102332. Epub 2022 Jan 22. Adv Healthc Mater. 2022. PMID: 35029040 Free PMC article.
-
Rolling Circular Amplification (RCA)-Assisted CRISPR/Cas9 Cleavage (RACE) for Highly Specific Detection of Multiple Extracellular Vesicle MicroRNAs.Anal Chem. 2020 Jan 21;92(2):2176-2185. doi: 10.1021/acs.analchem.9b04814. Epub 2020 Jan 9. Anal Chem. 2020. PMID: 31875674
-
Methods for Extracellular Vesicle Isolation: Relevance for Encapsulated miRNAs in Disease Diagnosis and Treatment.Genes (Basel). 2025 Mar 12;16(3):330. doi: 10.3390/genes16030330. Genes (Basel). 2025. PMID: 40149481 Free PMC article. Review.
-
Extracellular Vesicle microRNA: A Promising Biomarker and Therapeutic Target for Respiratory Diseases.Int J Mol Sci. 2024 Aug 23;25(17):9147. doi: 10.3390/ijms25179147. Int J Mol Sci. 2024. PMID: 39273095 Free PMC article. Review.
References
-
- a) Badimon L., Suades R., Vilella‐Figuerola A., Crespo J., Vilahur G., Escate R., Padro T., Chiva‐Blanch G., Antioxid. Redox. Signal 2020, 33, 645; - PubMed
- b) Chiva‐Blanch G., Padro T., Alonso R., Crespo J., Perez de Isla L., Mata P., Badimon L., Arterioscler Thromb. Vasc. Biol. 2019, 39, 945. - PubMed
-
- a) Gurunathan S., Kang M. H., Qasim M., Khan K., Kim J. H., Int. J. Nanomed. 2021, 16, 3357; - PMC - PubMed
- b) Simeone P., Bologna G., Lanuti P., Pierdomenico L., Guagnano M. T., Pieragostino D., Del Boccio P., Vergara D., Marchisio M., Miscia S., Mariani‐Costantini R., Int. J. Mol. Sci. 2020, 21, 2514; - PMC - PubMed
- c) Palviainen M., Saraswat M., Varga Z., Kitka D., Neuvonen M., Puhka M., Joenvaara S., Renkonen R., Nieuwland R., Takatalo M., Siljander P. R. M., PLoS One 2020, 15, 0236439; - PMC - PubMed
- d) Revenfeld A. L., Baek R., Nielsen M. H., Stensballe A., Varming K., Jorgensen M., Clin. Ther. 2014, 36, 830. - PubMed
-
- a) Price N. L., Rotllan N., Canfran‐Duque A., Zhang X., Pati P., Arias N., Moen J., Mayr M., Ford D. A., Baldan A., Suarez Y., Fernandez‐Hernando C., Cell Rep. 2017, 21, 1317; - PMC - PubMed
- b) Martinez‐Arroyo O., Ortega A., Flores‐Chova A., Sanchez‐Garcia B., Garcia‐Garcia A. B., Chaves F. J., Martin‐Escudero J. C., Forner M. J., Redon J., Cortes R., Eur. J. Intern. Med. 2023, 113, 49; - PMC - PubMed
- c) Leistner D. M., Boeckel J. N., Reis S. M., Thome C. E., De Rosa R., Keller T., Palapies L., Fichtlscherer S., Dimmeler S., Zeiher A. M., Eur. Heart J. 2016, 37, 1738. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

