HR-MS Analysis of the Covalent Binding of Edaravone to 5-Formylpyrimidine Bases and a DNA Oligonucleotide Containing a 5-Formylcytidine Residue
- PMID: 40255098
- PMCID: PMC12010150
- DOI: 10.1002/rcm.10050
HR-MS Analysis of the Covalent Binding of Edaravone to 5-Formylpyrimidine Bases and a DNA Oligonucleotide Containing a 5-Formylcytidine Residue
Abstract
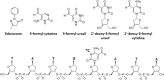
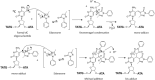
Rationale: Edaravone (EDA) is a radical scavenger and an antioxidant drug approved to treat amyotrophic lateral sclerosis and used as a research tool to explore treatment of neurodegenerative diseases and cancers. It is also a reactive agent, known as PMP (1-phenyl-3-methyl-5-pyrazolone), used for the analysis of polysaccharides composition. EDA can react with sugars and aromatic aldehydes. In this context, we have investigated the reactivity of EDA toward the biologically relevant formylated nucleobases, nucleosides, and an oligonucleotide containing a formylated residue.
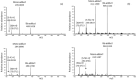
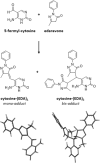
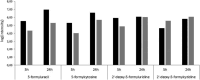
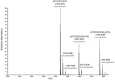
Methods: The formation of both mono- and bis-adducts between EDA and the formylated nucleobases (5-formyluracil (5fU) and 5-formylcytosine (5fC)) or the corresponding nucleosides 5-fdU and 5-fdC was characterized using high-resolution mass spectrometry (HR-MS). Similarly, the covalent binding of EDA to an 8-mer palindromic oligonucleotide d (TATG[*C]ATA) containing a single 5-fdC residue [*C] under physiological conditions was investigated using mass spectrometry.
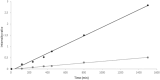
Results: For the first time, EDA is shown to react with formylated pyrimidines. Covalent and stable adducts were identified. EDA was found to react efficiently with the formylated oligonucleotide to generate mono- and bis-adducts. The rate of formation of the mono-adduct was five times higher than that of the bis-adduct. The reaction of EDA with aldehydic DNA modifications such as 5fU/5fC may have important consequences in terms of gene expression.
Conclusions: These observations raise implications for an epigenetic contribution to the mechanism of action of EDA. The biological implications of our in vitro results are discussed, notably in the frame of neurodegenerative diseases and cancers.
Keywords: Edaravone; covalent adducts; epigenetic; nucleosides; oligonucleotide.
© 2025 The Author(s). Rapid Communications in Mass Spectrometry published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Chemical reactivity and uses of 1-phenyl-3-methyl-5-pyrazolone (PMP), also known as edaravone.Bioorg Med Chem. 2020 May 15;28(10):115463. doi: 10.1016/j.bmc.2020.115463. Epub 2020 Mar 25. Bioorg Med Chem. 2020. PMID: 32241621 Review.
-
Antioxidant Properties and Aldehyde Reactivity of PD-L1 Targeted Aryl-Pyrazolone Anticancer Agents.Molecules. 2022 May 21;27(10):3316. doi: 10.3390/molecules27103316. Molecules. 2022. PMID: 35630791 Free PMC article.
-
Detection and Application of 5-Formylcytosine and 5-Formyluracil in DNA.Acc Chem Res. 2019 Apr 16;52(4):1016-1024. doi: 10.1021/acs.accounts.8b00543. Epub 2019 Jan 22. Acc Chem Res. 2019. PMID: 30666870
-
Potential use of edaravone to reduce specific side effects of chemo-, radio- and immuno-therapy of cancers.Int Immunopharmacol. 2019 Dec;77:105967. doi: 10.1016/j.intimp.2019.105967. Epub 2019 Oct 26. Int Immunopharmacol. 2019. PMID: 31670091 Review.
-
Edaravone inhibits protein carbonylation by a direct carbonyl-scavenging mechanism: focus on reactivity, selectivity, and reaction mechanisms.Antioxid Redox Signal. 2010 Mar;12(3):381-92. doi: 10.1089/ars.2009.2814. Antioxid Redox Signal. 2010. PMID: 19722825
References
-
- Wei Y., Zhong S., Yang H., et al., “Current Therapy in Amyotrophic Lateral Sclerosis (ALS): A Review on Past and Future Therapeutic Strategies,” European Journal of Medicinal Chemistry 272 (2024): 116496. - PubMed
-
- Johnson S. A., Fang T., De Marchi F., et al., “Pharmacotherapy for Amyotrophic Lateral Sclerosis: A Review of Approved and Upcoming Agents,” Drugs 82 (2022): 1367–1388. - PubMed
-
- Singh P., Belliveau P., Towle J., Neculau A. E., and Dima L., “Edaravone Oral Suspension: A Neuroprotective Agent to Treat Amyotrophic Lateral Sclerosis,” American Journal of Therapeutics 31 (2024): e258–e267. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
