Ionic liquid-iontophoresis mediates transdermal delivery of sparingly soluble drugs
- PMID: 40255114
- PMCID: PMC12013143
- DOI: 10.1080/10717544.2025.2489730
Ionic liquid-iontophoresis mediates transdermal delivery of sparingly soluble drugs
Abstract
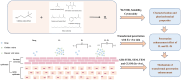
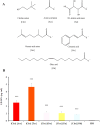
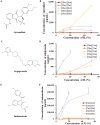
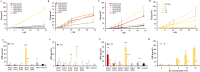
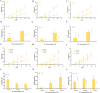
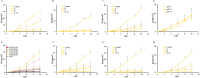
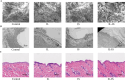
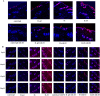
Low solubility restricted transdermal penetration of drugs. We aimed to develop a novel ionic liquid-iontophoresis (IL-IS) technology and assess their efficacy and primary factors in facilitating transdermal drug delivery. Five choline-based ILs with different chain length were synthesized and validated, and the impact of IL and/or IS technology on transdermal penetration of model drugs were investigated. The results indicated that five groups of ILs synthesized in this study exhibited minimal level of toxicity, and the longer the chain of acid ligands of ILs, the greater the cytotoxicity. The longer chain of acid ligand was demonstrated superior solubilizing capabilities compared to the shorter chain. Cinnamic acid-choline-based IL ([Cho] [Cin]) significantly improved permeation of all three model drugs, and permeation quantity was linearly positively associated with the concentration of ILs. The 10 h cumulative permeation of aripiprazole applied with ILs alone was enhanced by about 14-fold when paired with IS, and the penetration was linearly positively associated with the concentration and current strength of the ILs. In vivo results indicated that IL and/or IS technology primarily facilitated drug penetration into the skin, with potential involvement of endocytosis in this process. This study demonstrated that [Cho] [Cin] exhibited a significant enhancement in the transdermal delivery of three sparingly soluble drugs. It further enhanced the transdermal permeation of weak base drug following with the combining IL and IS technology. These findings highlighted that the IL-IS technology holded promise for facilitating the transdermal delivery of sparingly soluble and weak base drugs.
Keywords: Ionic liquids; iontophoresis; permeation enhancer; sparingly soluble; transdermal delivery.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Choline- versus imidazole-based ionic liquids as functional ingredients in topical delivery systems: cytotoxicity, solubility, and skin permeation studies.Drug Dev Ind Pharm. 2017 Nov;43(11):1858-1865. doi: 10.1080/03639045.2017.1349788. Epub 2017 Jul 12. Drug Dev Ind Pharm. 2017. PMID: 28665154
-
Choline and amino acid based biocompatible ionic liquid mediated transdermal delivery of the sparingly soluble drug acyclovir.Int J Pharm. 2020 May 30;582:119335. doi: 10.1016/j.ijpharm.2020.119335. Epub 2020 Apr 18. Int J Pharm. 2020. PMID: 32311469
-
Enhanced transdermal delivery of insulin by choline-based ionic liquids.Int J Pharm. 2024 Dec 25;667(Pt B):125006. doi: 10.1016/j.ijpharm.2024.125006. Epub 2024 Nov 26. Int J Pharm. 2024. PMID: 39603435
-
Design Principles and Applications of Ionic Liquids for Transdermal Drug Delivery.Adv Sci (Weinh). 2024 Nov;11(43):e2405983. doi: 10.1002/advs.202405983. Epub 2024 Sep 29. Adv Sci (Weinh). 2024. PMID: 39342651 Free PMC article. Review.
-
Recent Developments in Ionic Liquid-Assisted Topical and Transdermal Drug Delivery.Pharm Res. 2022 Oct;39(10):2335-2351. doi: 10.1007/s11095-022-03322-x. Epub 2022 Jul 1. Pharm Res. 2022. PMID: 35773446 Review.
References
-
- Arellano, H., et al. , 2025. Influence of critical micelle concentration of choline-based long chain fatty acid soaps on their antibacterial activity against methicillin resistant Staphylococcus aureus. Journal of colloid and interface science, 677 (Pt A), 314–323. doi: 10.1016/j.jcis.2024.07.218. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
