Evidence for a Modulatory Effect of a 12-Week Pomegranate Juice Intervention on the Transcriptional Response in Inflammatory Bowel Disease Patients Reducing Fecal Calprotectin Levels: Findings From a Proof-of-Principle Study
- PMID: 40255128
- PMCID: PMC12319513
- DOI: 10.1002/mnfr.70067
Evidence for a Modulatory Effect of a 12-Week Pomegranate Juice Intervention on the Transcriptional Response in Inflammatory Bowel Disease Patients Reducing Fecal Calprotectin Levels: Findings From a Proof-of-Principle Study
Abstract
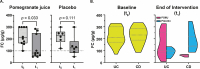
This study aimed at investigating the effects of pomegranate juice (POMJ) consumption on inflammatory biomarkers and gene expression in patients with inflammatory bowel disease (IBD) in clinical remission. In this randomized, placebo-controlled trial, 16 subjects with IBD in remission consumed POMJ or placebo for 12 weeks. POMJ consumption significantly reduced fecal calprotectin (FC) and plasma endotoxin levels. Transcriptomic analysis of peripheral blood mononuclear cells revealed upregulation of genes involved in mucosal immunity, including aryl hydrocarbon receptor (AHR), neutrophil cytosolic factor 4 (NCF4), and nuclear factor, interleukin 3 regulated (NFIL3). Urolithin metabotypes were predominantly of the B type, associated with intestinal dysbiosis. No significant changes were observed in serum inflammatory markers or colonic mucosal cytokine expression. POMJ consumption reduced markers of intestinal inflammation and modulated gene expression related to mucosal immunity and barrier function in patients with IBD. These findings suggest the potential of POMJ as a beneficial dietary intervention for maintaining remission in IBD, highlighting the promise of targeted nutritional strategies in managing chronic inflammatory conditions. Further research is needed to elucidate the long-term clinical implications of these molecular changes. TRIAL REGISTRATION: ClinicalTrials.gov identifier: NCT03000101.
Keywords: ellagitannins; fecal calprotectin; inflammatory bowel disease; pomegranate juice; urolithins.
© 2025 The Author(s). Molecular Nutrition & Food Research published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Pomegranate juice to reduce fecal calprotectin levels in inflammatory bowel disease patients with a high risk of clinical relapse: Study protocol for a randomized controlled trial.Trials. 2019 Jun 6;20(1):327. doi: 10.1186/s13063-019-3321-8. Trials. 2019. PMID: 31171016 Free PMC article.
-
Intestinal biomarkers, microbiota composition, and genetic predisposition to inflammatory bowel disease as predictors of Parkinson's disease manifestation.J Parkinsons Dis. 2025 Jun;15(4):766-779. doi: 10.1177/1877718X251328567. Epub 2025 May 7. J Parkinsons Dis. 2025. PMID: 40336252
-
Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation.Health Technol Assess. 2013 Nov;17(55):xv-xix, 1-211. doi: 10.3310/hta17550. Health Technol Assess. 2013. PMID: 24286461 Free PMC article.
-
Prebiotics for induction and maintenance of remission in ulcerative colitis.Cochrane Database Syst Rev. 2024 Mar 19;3(3):CD015084. doi: 10.1002/14651858.CD015084.pub2. Cochrane Database Syst Rev. 2024. PMID: 38501688 Free PMC article.
-
Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease.Inflamm Bowel Dis. 2006 Jun;12(6):524-34. doi: 10.1097/00054725-200606000-00013. Inflamm Bowel Dis. 2006. PMID: 16775498
References
-
- Le Berre C., Ananthakrishnan A. N., Danese S., Singh S., and Peyrin‐Biroulet L., “Ulcerative Colitis and Crohn's Disease Have Similar Burden and Goals for Treatment,” Clinical Gastroenterology and Hepatology 18 (2020): 14–23. - PubMed
-
- Larrosa M., González‐Sarrías A., Yáñez‐Gascón M. J., et al., “Anti‐inflammatory Properties of a Pomegranate Extract and Its Metabolite Urolithin‐A in a Colitis Rat Model and the Effect of Colon Inflammation on Phenolic Metabolism,” Journal of Nutritional Biochemistry 21 (2010): 717–725. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

