Recycling and Degradation Pathways of Synthetic Textile Fibers such as Polyamide and Elastane
- PMID: 40255241
- PMCID: PMC12003217
- DOI: 10.1002/gch2.202400163
Recycling and Degradation Pathways of Synthetic Textile Fibers such as Polyamide and Elastane
Abstract
Synthetic textile production is a major contributor to global waste growth, a phenomenon exacerbated by population growth and increased consumption. Global fiber production is expected to reach 147 million tons by 2030. New insights into recycling solutions are being developed. For example, progress has been made in recycling fibers such as polyester, including polyethylene terephthalate (PET), through the use of enzymes that can break specific bonds and return the material to its original state. However, this process must be carried out according to the nature of the polymer in question. In addition, the mixing of different synthetic fibers and the use of dyes make it difficult to develop a complete recycling process that separates the fibers and returns them to their original raw material. This review focuses on two types of fibers widely used in the textile industry, Nylon or polyamide (PA) and elastane (Spandex or Lycra), and explores the challenges and opportunities associated with their recycling.
Keywords: biotechnology; chemical recycling; elastane; polyamide; textile fibers; thermo‐mechanical recycling.
© 2025 The Author(s). Global Challenges published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
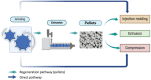
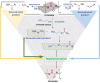
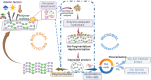

Similar articles
-
Recycled PET/PA6 Fibers from Waste Textile with Improved Hydrophilicity by In-Situ Reaction-Induced Capacity Enhancement.Polymers (Basel). 2024 Apr 11;16(8):1052. doi: 10.3390/polym16081052. Polymers (Basel). 2024. PMID: 38674974 Free PMC article.
-
Developing a compression moulded thermal insulation panel using postindustrial textile waste.Waste Manag. 2018 Sep;79:356-361. doi: 10.1016/j.wasman.2018.08.001. Epub 2018 Aug 7. Waste Manag. 2018. PMID: 30343764
-
Advancing Textile Waste Recycling: Challenges and Opportunities Across Polymer and Non-Polymer Fiber Types.Polymers (Basel). 2025 Feb 26;17(5):628. doi: 10.3390/polym17050628. Polymers (Basel). 2025. PMID: 40076120 Free PMC article. Review.
-
Recycling of Waste Cotton Textile Containing Elastane Fibers through Dissolution and Regeneration.Membranes (Basel). 2022 Mar 24;12(4):355. doi: 10.3390/membranes12040355. Membranes (Basel). 2022. PMID: 35448324 Free PMC article.
-
The Current State-of-the-Art of the Processes Involved in the Chemical Recycling of Textile Waste.Molecules. 2025 Jan 13;30(2):299. doi: 10.3390/molecules30020299. Molecules. 2025. PMID: 39860169 Free PMC article. Review.
References
-
- Climate trade, The world's most polluting industries, can be found under https://climatetrade.com/the‐worlds‐most‐polluting‐industries/ (accessed: May 2023).
-
- Parliament E., The Impact of Textile Production and Waste on the Environment (Infographics), 2020.
-
- Sonak I., Budget 2021–22: How sustainable is push for nylon, can be found under https://www.downtoearth.org.in/news/environment/budget‐2021‐22‐how‐susta... (accessed: February 2021).
-
- Exchange T., Materials Market Report, 2023.
Publication types
LinkOut - more resources
Full Text Sources

