Co-incubation of Short Amphiphilic Peptides with Dicer Substrate RNAs Results in β-Sheet Fibrils for Enhanced Gene Silencing in Cancer Cells
- PMID: 40255273
- PMCID: PMC12007892
- DOI: 10.59566/isrnn.2024.0101061
Co-incubation of Short Amphiphilic Peptides with Dicer Substrate RNAs Results in β-Sheet Fibrils for Enhanced Gene Silencing in Cancer Cells
Abstract
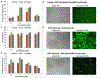
RNA can interact with positively charged, amphiphilic peptides to cooperatively assemble into fibrils that enable RNA transport across cancer cellular membranes. RNA decreases the folding energy barrier imposed by the electrostatic repulsion between these charged peptides, thus partaking in RNA-peptide self-assembly along particular pathways in the energy landscape. Specific amphiphilic peptides capable of protecting and transporting RNA across a membrane have Type II' β-turn hairpin forming motifs in their structures, which aids self-assembly into β-sheet fibrils. We employed a set of such cationic, amphiphilic peptides that have random coiled structures in the absence of folding stimuli, to characterize the (peptides):(RNA) assembly. We subjected these complexes to extensive biophysical characterization in vitro and in cell culture. We show that short RNAs (such as Dicer substrate RNAs) can lead these peptides to self-assemble into β-sheet fibrils that have RNA transport capabilities and can act as non-viral delivery vectors for RNA. Modulation in the peptide sequence implicitly alters the way they bind RNA and influence the peptides' ability to transport nucleic acids across membranes.
Keywords: Dicer substrate RNA; RNA interference; RNA-peptide co-evolution; amphiphilic peptides; cancer; peptide folding energy landscape; β-sheet fibrils.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflict of interest.
Figures





References
-
- Li M Z J, Lv Z, Qin H, Wang X, Shi H. Recent Advances in RNA-Targeted Cancer Therapy. Chembiochem. 2024; 25 (4). - PubMed
-
- Khalil IA, Sato Y, Harashima H. Recent advances in the targeting of systemically administered non-viral gene delivery systems. Expert Opin Drug Deliv. 2019; 16 (10): 1037–1050. - PubMed
-
- Pereira-Silva M, Jarak I, Alvarez-Lorenzo C, et al. Micelleplexes as nucleic acid delivery systems for cancer-targeted therapies. J Control Release. 2020; 323: 442–462. - PubMed
-
- Setten RL, Rossi JJ, Han SP. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019; 18 (6): 421–446. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
