Hidden β-γ Dehydrogenation Products in Long-Chain Fatty Acid Oxidation Unveiled by NMR: Implications on Lipid Metabolism
- PMID: 40255280
- PMCID: PMC12006827
- DOI: 10.1021/acsbiomedchemau.4c00140
Hidden β-γ Dehydrogenation Products in Long-Chain Fatty Acid Oxidation Unveiled by NMR: Implications on Lipid Metabolism
Abstract
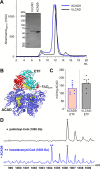
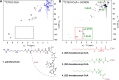
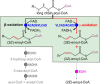
We present a comprehensive analysis of the initial α,β-dehydrogenation step in long-chain fatty acid β-oxidation (FAO). We focused on palmitoyl-CoA oxidized by two mitochondrial acyl-CoA dehydrogenases, very-long-chain acyl-CoA dehydrogenase (VLCAD) and acyl-CoA dehydrogenase family member 9 (ACAD9), both implicated in mitochondrial diseases. By combining MS and NMR, we identified the (2E)-hexadecenoyl-CoA as the expected α-β-dehydrogenation product and also the E and Z stereoisomers of 3-hexadecenoyl-CoA: a "γ-oxidation" product. This finding reveals an alternative catalytic pathway in mitochondrial FAO, suggesting a potential regulatory role for ACAD9 and VLCAD during fatty acid metabolism.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
A heterozygous missense mutation in adolescent-onset very long-chain acyl-CoA dehydrogenase deficiency with exercise-induced rhabdomyolysis.Tohoku J Exp Med. 2015 Apr;235(4):305-10. doi: 10.1620/tjem.235.305. Tohoku J Exp Med. 2015. PMID: 25843429
-
A new genetic disorder in mitochondrial fatty acid beta-oxidation: ACAD9 deficiency.Am J Hum Genet. 2007 Jul;81(1):87-103. doi: 10.1086/519219. Epub 2007 Jun 4. Am J Hum Genet. 2007. PMID: 17564966 Free PMC article.
-
Tissue-specific strategies of the very-long chain acyl-CoA dehydrogenase-deficient (VLCAD-/-) mouse to compensate a defective fatty acid β-oxidation.PLoS One. 2012;7(9):e45429. doi: 10.1371/journal.pone.0045429. Epub 2012 Sep 14. PLoS One. 2012. PMID: 23024820 Free PMC article.
-
Peroxisomal lipid degradation via beta- and alpha-oxidation in mammals.Cell Biochem Biophys. 2000;32 Spring:73-87. doi: 10.1385/cbb:32:1-3:73. Cell Biochem Biophys. 2000. PMID: 11330072 Review.
-
Mutation analysis in mitochondrial fatty acid oxidation defects: Exemplified by acyl-CoA dehydrogenase deficiencies, with special focus on genotype-phenotype relationship.Hum Mutat. 2001 Sep;18(3):169-89. doi: 10.1002/humu.1174. Hum Mutat. 2001. PMID: 11524729 Review.
References
-
- Honda A.; Nozumi M.; Ito Y.; Natsume R.; Kawasaki A.; Nakatsu F.; Abe M.; Uchino H.; Matsushita N.; Ikeda K.; Arita M.; Sakimura K.; Igarashi M. Very-long-chain fatty acids are crucial to neuronal polarity by providing sphingolipids to lipid rafts. Cell Reports 2023, 42 (10), 113195.10.1016/j.celrep.2023.113195. - DOI - PubMed
-
- Leslie N.; Wang X.; Peng Y.; Valencia C. A.; Khuchua Z.; Hata J.; Witte D.; Huang T.; Bove K. E. Neonatal multiorgan failure due to ACAD9 mutation and complex I deficiency with mitochondrial hyperplasia in liver, cardiac myocytes, skeletal muscle, and renal tubules. Human Pathology 2016, 49, 27–32. 10.1016/j.humpath.2015.09.039. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
