Recent advances in the design of SERS substrates and sensing systems for (bio)sensing applications: Systems from single cell to single molecule detection
- PMID: 40255478
- PMCID: PMC12009482
- DOI: 10.12688/f1000research.149263.3
Recent advances in the design of SERS substrates and sensing systems for (bio)sensing applications: Systems from single cell to single molecule detection
Abstract
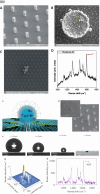
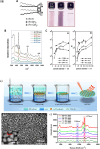
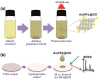
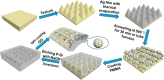
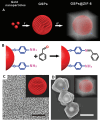
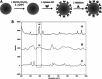
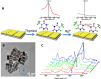
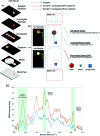
The Raman effect originates from spontaneous inelastic scattering of photons by matter. These photons provide a characteristic fingerprint of this matter, and are extensively utilized for chemical and biological sensing. However, the phenomenon do not hold potential for its direct use in sensing applications, since the generation of the Raman scattered photons is inherently low. Surface enhanced Raman spectroscopy (SERS) overcomes the low sensitivity associated with Raman spectroscopy and assists the sensing of diverse analytes, including ions, small molecules, inorganics, organics, radionucleotides, and cells. Plasmonic nanoparticles exhibit localized surface plasmon resonance (LSPR) and when they are closely spaced, they create hotspots where the electromagnetic field is significantly enhanced. This amplifies the Raman signal and may offer up to a 10 14-fold SERS signal enhancement. The development of SERS active substrates requires further consideration and optimization of several critical features such as surface periodicity, hotspot density, mitigation of sample or surface autofluorescence, tuning of surface hydrophilicities, use of specific (bio) recognition elements with suitable linkers and bioconjugation chemistries, and use of appropriate optics to obtain relevant sensing outcomes in terms of sensitivity, cross-sensitivity, limit of detection, signal-to-noise ratio (SNR), stability, shelf-life, and disposability. This article comprehensively reviews the recent advancements on the use of disposable materials such as commercial grades of paper, textiles, glasses, polymers, and some specific substrates such as blue-ray digital versatile discs (DVDs) for use as SERS-active substrates for point-of-use (POU) sensing applications. The advancements in these technologies have been reviewed and critiqued for analyte detection in resource-limited settings, highlighting the prospects of applications ranging from single-molecule to single-cell detection. We conclude by highlighting the prospects and possible avenues for developing viable field deployable sensors holding immense potential in environmental monitoring, food safety and biomedical diagnostics.
Keywords: Bioreceptor.; Disposable substrates; Point-of-use; Raman effect; Single-molecule sensing; Surface Enhanced Raman Spectroscopy.
Copyright: © 2025 Tadi SR et al.
Conflict of interest statement
No competing interests were disclosed.
Figures











Similar articles
-
Large-Area Nanogap Platforms for Surface-Enhanced Raman Spectroscopy Toward Sensing Applications: Comparison Between Ag and Au.Biosensors (Basel). 2025 Jun 9;15(6):369. doi: 10.3390/bios15060369. Biosensors (Basel). 2025. PMID: 40558451 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article.
References
-
- Abidin U, Daud NASM, Le Brun V: Replication and leakage test of polydimethylsiloxane (PDMS) microfluidics channel. 2019;020064. 10.1063/1.5086611 - DOI
-
- Agarwal NR, Lucotti A, Tommasini M, et al. : SERS detection and DFT calculation of 2-naphthalene thiol adsorbed on Ag and Au probes. Sensors Actuators B Chem. 2016;237:545–555. 10.1016/j.snb.2016.06.143 - DOI
-
- Akagi T, Baba M, Akashi M: Preparation of nanoparticles by the self-organization of polymers consisting of hydrophobic and hydrophilic segments: Potential applications. Polymer. 2007;48(23):6729–6747. 10.1016/j.polymer.2007.08.038 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

