Nucleic Acid-Functionalized Gold Nanorods Modulate Inflammation and Dysregulated Intestinal Barriers for Treatment of Ulcerative Colitis
- PMID: 40255504
- PMCID: PMC12006742
- DOI: 10.34133/bmr.0195
Nucleic Acid-Functionalized Gold Nanorods Modulate Inflammation and Dysregulated Intestinal Barriers for Treatment of Ulcerative Colitis
Abstract
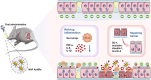
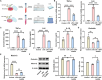
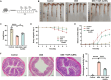
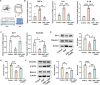
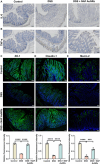
Traditional oral treatments for ulcerative colitis (UC) face marked limitations including their single therapeutic effect, potential off-target interactions, and toxic side effects. In this study, we present nucleic acid-functionalized gold nanorods (NAF AuNRs), a biocompatible nanomaterial designed for the oral treatment of dextran sulfate sodium (DSS)-induced colitis. The NAF AuNRs alleviate immune responses by inhibiting pro-inflammatory macrophages and enhancing the expression of barrier proteins in intestinal epithelial cells. Due to the negatively charged nucleic acid shell, NAF AuNRs preferentially target anionic, inflamed colon tissues upon oral administration, reducing pro-inflammatory cytokine levels and promoting the recovery of intestinal barrier in DSS-induced colitis mice. Collectively, these findings suggest that NAF AuNRs represent an innovative and promising therapeutic approach for UC management, offering novel insights into the application of nucleic acid-functionalized nanomaterials.
Copyright © 2025 Wanghong He et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures

Similar articles
-
Resveratrol alleviates intestinal mucosal barrier dysfunction in dextran sulfate sodium-induced colitis mice by enhancing autophagy.World J Gastroenterol. 2020 Sep 7;26(33):4945-4959. doi: 10.3748/wjg.v26.i33.4945. World J Gastroenterol. 2020. PMID: 32952341 Free PMC article.
-
Fufangxiaopi formula alleviates DSS-induced colitis in mice by inhibiting inflammatory reaction, protecting intestinal barrier and regulating intestinal microecology.J Ethnopharmacol. 2024 Jan 30;319(Pt 3):117365. doi: 10.1016/j.jep.2023.117365. Epub 2023 Oct 30. J Ethnopharmacol. 2024. PMID: 38380568
-
Celecoxib alleviates the DSS-induced ulcerative colitis in mice by enhancing intestinal barrier function, inhibiting ferroptosis and suppressing apoptosis.Immunopharmacol Immunotoxicol. 2024 Apr;46(2):240-254. doi: 10.1080/08923973.2023.2300508. Epub 2024 Jan 18. Immunopharmacol Immunotoxicol. 2024. PMID: 38156770
-
Anti-inflammatory effects of Brucea javanica oil emulsion by suppressing NF-κB activation on dextran sulfate sodium-induced ulcerative colitis in mice.J Ethnopharmacol. 2017 Feb 23;198:389-398. doi: 10.1016/j.jep.2017.01.042. Epub 2017 Jan 22. J Ethnopharmacol. 2017. PMID: 28119098
-
Protopine Alleviates Dextran Sodium Sulfate-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Regulating Intestinal Microbiota.Molecules. 2023 Jul 7;28(13):5277. doi: 10.3390/molecules28135277. Molecules. 2023. PMID: 37446938 Free PMC article.
References
-
- Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet. 2023;402(10401):571–584. - PubMed
-
- Wang M, Cha R, Hao W, Jiang X. Nanocrystalline cellulose modulates dysregulated intestinal barriers in ulcerative colitis. ACS Nano. 2023;17(19):18965–18978. - PubMed
-
- Huang Q, Yang Y, Zhu Y, Chen Q, Zhao T, Xiao Z, Wang M, Song X, Jiang Y, Yang Y, et al. . Oral metal-free melanin nanozymes for natural and durable targeted treatment of inflammatory bowel disease (IBD). Small. 2023;19(19): Article e2207350. - PubMed
LinkOut - more resources
Full Text Sources

