A Phase 2, Multi-Center, Randomized, Double-Blind, Parallel-Group Trial to Evaluate the Efficacy and Safety of CKD-495 in Patients With Acute and Chronic Gastritis
- PMID: 40255536
- PMCID: PMC12006708
- DOI: 10.1155/cjgh/2702089
A Phase 2, Multi-Center, Randomized, Double-Blind, Parallel-Group Trial to Evaluate the Efficacy and Safety of CKD-495 in Patients With Acute and Chronic Gastritis
Abstract
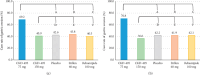
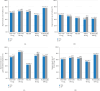
CKD-495 is a newly developed drug extracted from Cinnamomum cassia Presl. This phase II study assessed the clinical benefits of CKD-495 in the treatment of acute and chronic gastritis. This study randomly assigned 250 patients with endoscopically-proven gastric mucosal erosion to five groups. The groups received either 75 mg or 150 mg of CKD-495, 100 mg of rebamipide, 60 mg of Artemisiae argyi folium 95% ethanol ext. (20 ⟶ 1) (Stillen; Dong-A ST Co., Ltd., Seoul, Korea), or placebo for 2 weeks, respectively. The primary endpoint was the erosion improvement rate, and the secondary endpoints were erosion cure rates, improvement rates of gastrointestinal symptoms, edema, redness, and hemorrhage. Drug-related adverse events were evaluated. The endoscopic erosion improvement rate was significantly higher in the 75 mg CKD-495 group than in the other groups in both the full analysis set (73% vs. 41%, 45%, 52%, 48% for the 75 mg CKD-495, 150 mg CKD-495, placebo, 60 mg Stillen, and 100 mg rebamipide groups, respectively) and the per-protocol set (PPS) (75% vs. 37%, 45%, 51%, 50%). The cure rate of gastric erosion was significantly higher in the 75 mg CKD-495 group than in the other groups. The improvement rates of hemorrhage erosion were significantly higher in the 150-mg CKD-495 group. No significant differences were observed in the safety profiles. No serious adverse events or drug reactions were observed. These results demonstrate that 75 mg of CKD-495 has excellent efficacy for the treatment of endoscopic and symptomatic improvements for acute and chronic gastritis. Trial Registration: ClinicalTrials.gov identifier: NCT03437785.
Copyright © 2025 Su Hyun Park et al. Canadian Journal of Gastroenterology and Hepatology published by John Wiley & Sons Ltd.
Conflict of interest statement
This study was supported by Chong Kun Dang Pharmaceutical Corp. (CKD), which provided research funding and investigational drugs. Author Shin Jung Park served as a research director at CKD from 2001 to 2023 and the authors acknowledge that this may present a potential conflict of interest. However, CKD had no involvement in data collection during the trial and played no role in writing or deciding to publish this article. Furthermore, this study was conducted in a double-blinded manner, ensuring that all data were collected without bias, and the final results and conclusions were based on the independent judgment of the authors, not the sponsor's affiliation.
Figures



References
-
- Talley N. J. Update on the Role of Drug Therapy in Non-Ulcer Dyspepsia. Reviews in Gastroenterological Disorders . 2003;3(1):25–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

