Eculizumab is efficacious and safe in pediatric patients with various forms of hemolytic uremic syndrome: a retrospective clinical experience of a tertiary center
- PMID: 40255570
- PMCID: PMC12006164
- DOI: 10.3389/fphar.2025.1535407
Eculizumab is efficacious and safe in pediatric patients with various forms of hemolytic uremic syndrome: a retrospective clinical experience of a tertiary center
Abstract
Background: Eculizumab, a terminal complement inhibitor, prevents thrombotic microangiopathy (TMA) and multiorgan damage in hemolytic uremic syndrome (HUS). We evaluated its efficacy and safety in pediatric patients with TMA sub-types: atypical HUS (aHUS), Shiga toxin-producing Escherichia coli (STEC)-HUS, and transplant-associated TMA (TA-TMA).
Methods: This retrospective study included all pediatric patients treated with eculizumab for HUS at Schneider Children's Medical Center (2011-2020), including those with pre-existing end-stage kidney disease. Clinical and laboratory parameters were analyzed over 28 weeks. The primary endpoint was achievement of complete TMA response, defined by sustained normalization of hematologic parameters and renal function. Secondary endpoints included TMA event-free status and additional clinical improvements.
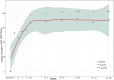
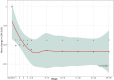
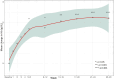
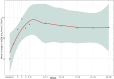
Results: Twenty-four pediatric patients (median age 5.8 years) were included: 13 with aHUS, 5 with STEC-HUS, and 6 with TA-TMA. A complete TMA response was achieved in 12 (50%) of the patients overall: 7 (54%) with aHUS, 3 (60%) with STEC-HUS, and 2 (33%) with TA-TMA. TMA event-free status was reached in 15 (63%) patients. Significant improvements were observed in platelet count (63%), lactate dehydrogenase levels (76% within the first week), hemoglobin (60%), and estimated glomerular filtration rate (79%); while CH-50 levels decreased. No severe adverse events were attributed to eculizumab. Chronic kidney disease stage improved for 17 (90%).
Conclusion: The efficacy and safety of eculizumab for three TMA subtypes in pediatric patients potentially expands its therapeutic applications. The complete TMA response rate in aHUS supports eculizumab as a first-line use, while the response rate in STEC-HUS suggests potential efficacy beyond eculizumab's primary indication. The early hematologic responses and reduced CH-50 levels confirm the role of eculizumab complement-mediated HUS and underscore the need for further research in TA-TMA.
Keywords: STEC-HUS; TA-TMA; aHUS; eculizumab; hemolytic uremic syndrome; pediatrics.
Copyright © 2025 Lax, Davidovits, Chodick, Bernfeld and Peled.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance.Clin Exp Nephrol. 2019 Jan;23(1):112-121. doi: 10.1007/s10157-018-1610-2. Epub 2018 Jul 23. Clin Exp Nephrol. 2019. PMID: 30039480 Free PMC article.
-
Efficacy and Safety of Eculizumab in Pediatric Patients Affected by Shiga Toxin-Related Hemolytic and Uremic Syndrome: A Randomized, Placebo-Controlled Trial.J Am Soc Nephrol. 2023 Sep 1;34(9):1561-1573. doi: 10.1681/ASN.0000000000000182. Epub 2023 Jun 12. J Am Soc Nephrol. 2023. PMID: 37303085 Free PMC article. Clinical Trial.
-
Terminal Complement Inhibitor Eculizumab in Adult Patients With Atypical Hemolytic Uremic Syndrome: A Single-Arm, Open-Label Trial.Am J Kidney Dis. 2016 Jul;68(1):84-93. doi: 10.1053/j.ajkd.2015.12.034. Epub 2016 Mar 21. Am J Kidney Dis. 2016. PMID: 27012908 Clinical Trial.
-
Natural history of thrombotic thrombocytopenic purpura and hemolytic uremic syndrome.Semin Thromb Hemost. 2014 Nov;40(8):866-73. doi: 10.1055/s-0034-1395154. Epub 2014 Nov 6. Semin Thromb Hemost. 2014. PMID: 25377323 Review.
-
Hemolytic Uremic Syndrome and Kidney Transplantation: A Case Series and Review of the Literature.Nephron. 2017;136(3):245-253. doi: 10.1159/000468528. Epub 2017 Apr 19. Nephron. 2017. PMID: 28419995 Review.
References
LinkOut - more resources
Full Text Sources

