Antiviral activity of eicosapentaenoic acid against zika virus and other enveloped viruses
- PMID: 40255573
- PMCID: PMC12006069
- DOI: 10.3389/fphar.2025.1564504
Antiviral activity of eicosapentaenoic acid against zika virus and other enveloped viruses
Abstract
Background: Zika virus (ZIKV) is an emerging flavivirus that may cause innate microcephaly or neurological disturbances. Yet no antiviral has been approved by FDA against ZIKV infection. It was shown that some unsaturated fatty acids could inactivate enveloped viruses including SARS-CoV-2. However, studies investigating the effect of eicosapentaenoic acid (EPA) on ZIKV infection are lacking. This study aims to evaluate the antiviral effect of EPA against ZIKV and other enveloped viruses.
Methods: We first explored the toxicities of EPA in vitro and in vivo. Then we examined the antiviral effect of EPA against ZIKV via cell-based immunodetection, qRT-PCR, Western blotting, and so on. To uncover its antiviral mechanism, we performed assays for virus binding, adsorption and entry, and time-of-addition. RNase digestion and ZIKV NS2B-NS3 protease inhibition assays were also adopted. Finally, we detected its effects on dengue virus (DENV)-2, herpes simplex virus (HSV)-1 and influenza A virus via MTT, Western blotting and qRT-PCR assays.
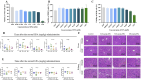
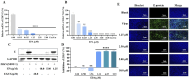
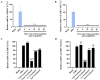
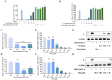
Results: EPA was found to inhibit ZIKV infection in vitro without causing cytotoxicities. EPA exhibited antiviral activity in the early stages of the ZIKV life cycle quickly. Mechanistic experiments showed that EPA disrupted the membrane integrity of viral particles, leading to the release of viral RNA, together with the interruption of ZIKV from binding, adsorption and entry, and ultimately the inhibition of viral proliferation. Furthermore, EPA exerted antiviral effects against DENV-2, HSV-1, and influenza virus, in a dose-dependent manner.
Conclusion: These findings suggest that EPA is a promising broad-spectrum antiviral drug candidate.
Keywords: EPA; ZIKV; adsorption; antiviral activity; binding; entry.
Copyright © 2025 Feng, Qiu, Zou, Li, Chen, Chen, Ma, Liu, Lai, Liu and Zou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Identification of alpha-linolenic acid as a broad-spectrum antiviral against zika, dengue, herpes simplex, influenza virus and SARS-CoV-2 infection.Antiviral Res. 2023 Aug;216:105666. doi: 10.1016/j.antiviral.2023.105666. Epub 2023 Jul 8. Antiviral Res. 2023. PMID: 37429528
-
Structure-Based Virtual Screening: Identification of a Novel NS2B-NS3 Protease Inhibitor with Potent Antiviral Activity against Zika and Dengue Viruses.Microorganisms. 2021 Mar 6;9(3):545. doi: 10.3390/microorganisms9030545. Microorganisms. 2021. PMID: 33800763 Free PMC article.
-
Viperin Restricts Zika Virus and Tick-Borne Encephalitis Virus Replication by Targeting NS3 for Proteasomal Degradation.J Virol. 2018 Mar 14;92(7):e02054-17. doi: 10.1128/JVI.02054-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29321318 Free PMC article.
-
Advances in Computational Methods to Discover New NS2B-NS3 Inhibitors Useful Against Dengue and Zika Viruses.Curr Top Med Chem. 2022;22(29):2435-2462. doi: 10.2174/1568026623666221122121330. Curr Top Med Chem. 2022. PMID: 36415099 Review.
-
NS2B-NS3 protease inhibitors as promising compounds in the development of antivirals against Zika virus: A systematic review.J Med Virol. 2022 Feb;94(2):442-453. doi: 10.1002/jmv.27386. Epub 2021 Oct 20. J Med Virol. 2022. PMID: 34636434
References
-
- Argaw A., Bouckaert K. P., Wondafrash M., Kolsteren P., Lachat C., Meulenaer B. D., et al. (2021). Effect of fish-oil supplementation on breastmilk long-chain polyunsaturated fatty acid concentration: a randomized controlled trial in rural Ethiopia. Eur. J. Clin. Nutr. 75 (5), 809–816. 10.1038/s41430-020-00798-x - DOI - PubMed
-
- Arsic A., Krstic P., Paunovic M., Nedovic J., Jakovljevic V., Vucic V. (2023). Anti-inflammatory effect of combining fish oil and evening primrose oil supplementation on breast cancer patients undergoing chemotherapy: a randomized placebo-controlled trial. Sci. Rep. 13 (1), 6449. 10.1038/s41598-023-28411-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

