Development of a decellularized extracellular matrix-derived wet adhesive for sustained drug delivery and enhanced wound healing
- PMID: 40255583
- PMCID: PMC12008594
- DOI: 10.1016/j.mtbio.2025.101734
Development of a decellularized extracellular matrix-derived wet adhesive for sustained drug delivery and enhanced wound healing
Abstract
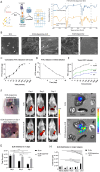
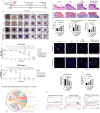
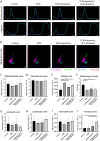
Complete tissue recovery following traumatic injury remains a major clinical challenge. While tissue adhesives show promise for managing traumatic injuries, developing materials with robust wet adhesion and high biocompatibility remains difficult. Decellularized extracellular matrix (ECM)-derived materials are widely utilized in tissue engineering due to their superior biocompatibility and bioactivity. In this study, a wet adhesive is developed by functionalizing ECM with dopamine. The resulting ECM-dopamine exhibits strong wet adhesion and excellent biocompatibility. Furthermore, ECM-dopamine can be engineered into a drug delivery platform for small agents and macromolecules. Solid lipid nanoparticles (SLNs) are incorporated into ECM-dopamine to enable sustained release of small molecules. The ECM-dopamine-SLN system ensures sustained drug release for at least one week upon adhesion to target tissues. ECM-dopamine-SLN loaded with antimicrobials accelerates wound healing and promotes angiogenesis by modulating the inflammatory response in a mouse skin excision model. Additionally, ECM-dopamine can deliver bioactive macromolecules to injured tissue. ECM-dopamine loaded with insulin-like growth factor-1 promotes skeletal muscle regeneration in a mouse volumetric muscle loss model, likely through the modulation of M2-like macrophage polarization. The dual functionality of ECM-dopamine as both a wet adhesive and a drug delivery platform offers significant potential for regenerative medicine applications.
Keywords: Drug delivery; Extracellular matrix derived-material; Volumetric muscle loss; Wet adhesion; Wound healing.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Mesenchymal stem cells and extracellular matrix scaffold promote muscle regeneration by synergistically regulating macrophage polarization toward the M2 phenotype.Stem Cell Res Ther. 2018 Apr 3;9(1):88. doi: 10.1186/s13287-018-0821-5. Stem Cell Res Ther. 2018. PMID: 29615126 Free PMC article.
-
Dopamine based adhesive nano-coatings on extracellular matrix (ECM) based grafts for enhanced host-graft interfacing affinity.Nanoscale. 2021 Nov 11;13(43):18148-18159. doi: 10.1039/d1nr06284k. Nanoscale. 2021. PMID: 34709280
-
Inflammation-mediated matrix remodeling of extracellular matrix-mimicking biomaterials in tissue engineering and regenerative medicine.Acta Biomater. 2022 Oct 1;151:106-117. doi: 10.1016/j.actbio.2022.08.015. Epub 2022 Aug 13. Acta Biomater. 2022. PMID: 35970482 Review.
-
Extracellular matrix component-derived nanoparticles for drug delivery and tissue engineering.J Control Release. 2023 Aug;360:888-912. doi: 10.1016/j.jconrel.2023.07.034. Epub 2023 Jul 26. J Control Release. 2023. PMID: 37482344
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
Cited by
-
ECM-inspired stem cell secretome sustained releasing composite nanofibrous membranes for accelerated wound healing.Mater Today Bio. 2025 Jul 26;34:102141. doi: 10.1016/j.mtbio.2025.102141. eCollection 2025 Oct. Mater Today Bio. 2025. PMID: 40791792 Free PMC article.
References
-
- Prevaldi C., Paolillo C., Locatelli C., Ricci G., Catena F., Ansaloni L., Cervellin G. Management of traumatic wounds in the emergency department: position paper from the academy of emergency medicine and Care (AcEMC) and the world society of emergency surgery (WSES) World J. Emerg. Surg. Jun. 2016;11(1):1–6. doi: 10.1186/S13017-016-0084-3/TABLES/4. - DOI - PMC - PubMed
-
- Nair A., Dahiya A., Yadav P., Sharma N., Butola B.S. Materials for the management of traumatic Wounds: a descriptive review. Eur. Polym. J. Jan. 2025;222 doi: 10.1016/J.EURPOLYMJ.2023.112475. - DOI
-
- Frantzis P. Durability of adhesive joints made underwater. J. Mater. Civ. Eng. Oct. 2008;20(10):635–639. doi: 10.1061/(ASCE)0899-1561(2008)20:10(635). - DOI
LinkOut - more resources
Full Text Sources

