A Novel Dynamic Compression Angle-Stable Interlocking Intramedullary Nail: Description, Validation, and Model Evaluation
- PMID: 40255609
- PMCID: PMC12006711
- DOI: 10.1155/vmi/7875699
A Novel Dynamic Compression Angle-Stable Interlocking Intramedullary Nail: Description, Validation, and Model Evaluation
Abstract
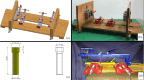
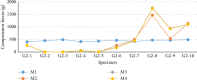
The stabilization of long-bone fractures using intramedullary nails offers significant biological advantages for bone healing. Nevertheless, the mechanical stability of the implant-bone interface remains suboptimal due to the absence of models capable of generating interfragmentary compression at the fracture site. To address these limitations, this study aims to describe and evaluate a novel dynamic compression angle-stable interlocking intramedullary nail (DCASIN), designed for use in conjunction with a compression device (CD). Its performance was compared with conventional and angle-stable interlocking intramedullary nails. Implantation was demonstrated using a tube-based bone model with transverse fractures. Compression was achieved in the proximal aspect of the DCASIN through an oblong hole that allowed the insertion of a Steinmann pin, which was then subjected to the thrust of the CD's primary screw (PS). To evaluate dynamic compression, a load cell connected to the Arduino/Genuíno Uno software was utilized. Three groups of interlocking nails were assessed: G1 (conventional), G2 (angle-stable), and G3 (DCASIN), with measurements taken at four time points (M1: prelocking, M2: after the first screw or PS for the DCASIN, M3: after the second implant, and M4: one-minute post-M3). No statistically significant differences in compression forces were observed for G1 and G2 across the measured time points. In contrast, G3 exhibited significantly higher compression at M2 than at M3 and M4, and its compression forces at M2, M3, and M4 were significantly greater than those in G1 and G2. Finite element analysis revealed no significant deformation in G3 during compression. In conclusion, the DCASIN combined with the CD achieved and sustained superior compression forces compared to conventional and angle-stable nails, thereby offering a promising alternative for the internal fixation of long bones.
Keywords: biological osteosynthesis; experimental implants; implants engineer; intramedullary fracture fixation; long bone fractures.
Copyright © 2025 Luís Gustavo Gosuen Gonçalves Dias et al. Veterinary Medicine International published by John Wiley & Sons Ltd.
Conflict of interest statement
Luís Gustavo Gosuen Gonçalves Dias and São Paulo State University (UNESP) had patent #BR 10 2018 016021 4 licensed to the Brazilian National Institute of Industrial Property. The other authors declare no conflicts of interest.
Figures











References
-
- Johnston S. A., von Pfeil D. J. F., Déjardin L. M., Weh M., Roe S. Internal Fracture Fixation. In: Tobias K. M., Johnston S. A., editors. Veterinary Surgery: Small Animal . Saint Louis: Elsevier; 2017. pp. 1892–1983.
-
- Maritato K. C. A Brief History of Veterinary Locking Plates Applications. In: Barnhart M. D., Maritato K. C., editors. Locking Plates in Veterinary Orthopedics . Hoboken: Wiley-Blackwell; 2019. pp. 1–5.
LinkOut - more resources
Full Text Sources

