Glucose-dependent insulinotropic polypeptide stimulates post-absorptive lipid secretion in the intestine
- PMID: 40255636
- PMCID: PMC12006050
- DOI: 10.3389/fphys.2025.1549392
Glucose-dependent insulinotropic polypeptide stimulates post-absorptive lipid secretion in the intestine
Abstract
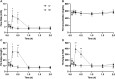
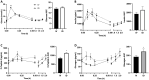
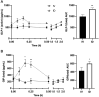
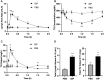
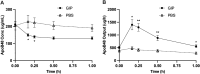
It is increasingly recognized that the intestine can retain a portion of dietary fats for secretion during the post-absorptive state, which has strong implications in metabolic diseases. The regulatory mechanisms of gut lipid storage and release are not well defined. Previous studies showed that the intestine releases locally stored fats in response to several stimulatory cues, such as glucose delivered into the intestinal lumen. It remains unknown how the intestine responds to nutrient signals in this phenomenon. Here we tested the effects of intravenous glucose delivery on intestinal lipid output during the post-absorptive state in mesenteric lymph duct cannulated rats. Compared with intraduodenal glucose delivery, intravenous glucose did not stimulate intestinal lipid output. Intraduodenal glucose was also associated with increases in blood levels of metabolic hormones, among which glucose-dependent insulinotropic peptide (GIP) levels were significantly higher at timepoints corresponding to increased lipid output than in intravenous glucose. Intraperitoneal GIP administration per se robustly stimulated intestinal lipid output. These results support a mechanism that involves glucose sensing at the apical side of the enterocytes and GIP as a potent stimulus for the release of lipid storage from the intestine.
Keywords: chylomicron; glucose; glucose-dependent insulinotropic peptide; intestine; triglycerides.
Copyright © 2025 Wang, Khan, Mukherjee, Ghanem and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Stimulation of incretin secretion by dietary lipid: is it dose dependent?Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G299-305. doi: 10.1152/ajpgi.90601.2008. Epub 2009 Jun 11. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19520739 Free PMC article.
-
Glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide stimulate release of substance P from TRPV1- and TRPA1-expressing sensory nerves.Am J Physiol Gastrointest Liver Physiol. 2020 Jul 1;319(1):G23-G35. doi: 10.1152/ajpgi.00189.2019. Epub 2020 May 18. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32421358 Free PMC article.
-
Chylomicron formation and secretion is required for lipid-stimulated release of incretins GLP-1 and GIP.Lipids. 2012 Jun;47(6):571-80. doi: 10.1007/s11745-011-3650-1. Lipids. 2012. PMID: 22297815 Free PMC article.
-
Emerging Role of Lymphatics in the Regulation of Intestinal Lipid Mobilization.Front Physiol. 2020 Jan 29;10:1604. doi: 10.3389/fphys.2019.01604. eCollection 2019. Front Physiol. 2020. PMID: 32063861 Free PMC article. Review.
-
Clinical endocrinology and metabolism. Glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide.Best Pract Res Clin Endocrinol Metab. 2004 Dec;18(4):587-606. doi: 10.1016/j.beem.2004.08.007. Best Pract Res Clin Endocrinol Metab. 2004. PMID: 15533777 Review.
References
-
- Coon S. D., Rajendran V. M., Schwartz J. H., Singh S. K. (2015). Glucose-dependent insulinotropic polypeptide-mediated signaling pathways enhance apical PepT1 expression in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G56–G62. 10.1152/ajpgi.00168.2014 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

