Gold and Silver Nanoparticles Efficiently Modulate the Crosstalk Between Macrophages and Cancer Cells
- PMID: 40255669
- PMCID: PMC12009049
- DOI: 10.2147/IJN.S508171
Gold and Silver Nanoparticles Efficiently Modulate the Crosstalk Between Macrophages and Cancer Cells
Abstract
Background: Macrophages, polarized into pro-inflammatory M1 or anti-inflammatory M2 states, are essential cellular elements of innate immunity. In the tumor microenvironment, owing to a paracrine manipulative program by cancerous cells, tumor-associated macrophages (TAMs) evolve, which can shift between M1-like and M2-like phenotypes. Since it is fairly unknown how the promising anticancer agents, silver (AgNPs) and gold nanoparticles (AuNPs) affect the bidirectional communication and reprogramming in the tumor stroma, we examined the behavior, the tumor-supporting functions, and the expression of polarization and functional marker genes of TAMs to reveal how these are modulated upon interaction with nanoparticle-exposed cancer cells.
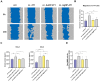
Methods: We established co-cultures of murine immortalized J774 or primary bone marrow-derived macrophages with 4T1 breast cancer cells treated with AuNPs or AgNPs or with none of the nanoparticles. We assessed the expression of macrophage polarization and functional markers using RT-qPCR and Proteome Profiler Array and evaluated macrophage migration and matrix metalloproteinase activity by specific assays.
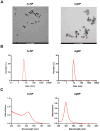
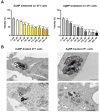
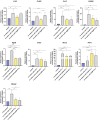
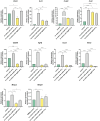
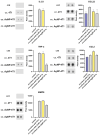
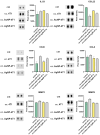
Results: Protein and mRNA levels of most examined factors - except tumor necrosis factor-alpha - such as C-C-motif chemokine ligands 2 and 22, interleukin-23, inducible nitric oxide synthase, cyclooxygenase-2, the macrophage mannose receptor CD206, transforming growth factor-beta, and chitinase-like-3 protein decreased, and the expression of polarization markers revealed a shift towards M1-like phenotype in macrophages co-cultured with AgNP- or AuNP-treated 4T1 cells. Both nanoparticle treatments reduced the levels and activity of cell migration-related factors, such as C-C motif chemokine ligand 3, matrix metalloproteinases, and suppressed macrophage migration.
Conclusion: Both AuNPs and AgNPs showed a remarkable ability to influence macrophage-cancer cell communication, suppressed indirectly M2-like TAM polarization, and perturbed the migration behavior of TAMs that is critical for tumor invasion, indicating modulated immunological functions and debilitated cancer-promoting capabilities of TAMs in this microenvironment.
Keywords: macrophage-tumor cell co-culture; metal nanoparticles; paracrine; tumor microenvironment; tumor-associated macrophage.
© 2025 Adamecz et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
F1 fraction isolated from Mesobuthus eupeus scorpion venom induces macrophage polarization toward M1 phenotype and exerts anti-tumoral effects on the CT26 tumor cell line.Int Immunopharmacol. 2024 May 10;132:111960. doi: 10.1016/j.intimp.2024.111960. Epub 2024 Mar 29. Int Immunopharmacol. 2024. PMID: 38554440
-
Protein-Bound Polysaccharides from Coriolus Versicolor Fungus Disrupt the Crosstalk Between Breast Cancer Cells and Macrophages through Inhibition of Angiogenic Cytokines Production and Shifting Tumour-Associated Macrophages from the M2 to M1 Subtype.Cell Physiol Biochem. 2020 Jun 20;54(4):615-628. doi: 10.33594/000000244. Cell Physiol Biochem. 2020. PMID: 32559360
-
Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2.BMC Cancer. 2018 May 22;18(1):579. doi: 10.1186/s12885-018-4299-4. BMC Cancer. 2018. PMID: 29783929 Free PMC article.
-
Biological Effects of Nanoparticles on Macrophage Polarization in the Tumor Microenvironment.Nanotheranostics. 2019 Jan 1;3(1):66-88. doi: 10.7150/ntno.30052. eCollection 2019. Nanotheranostics. 2019. PMID: 30662824 Free PMC article. Review.
-
Nanotherapeutics for Macrophage Network Modulation in Tumor Microenvironments: Targets and Tools.Int J Nanomedicine. 2024 Dec 19;19:13615-13651. doi: 10.2147/IJN.S491573. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39717515 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

