Neurophysiological signatures of ageing: compensatory and compromised neural mechanisms
- PMID: 40255691
- PMCID: PMC12006661
- DOI: 10.1093/braincomms/fcaf131
Neurophysiological signatures of ageing: compensatory and compromised neural mechanisms
Abstract
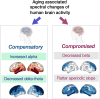
Spatiotemporal patterns of neural oscillations change with ageing, even in the cognitively unimpaired individual. Whether these neurophysiological changes represent ageing-related vulnerabilities or mechanisms that support cognitive resilience remains largely unknown. In this study, we used magnetoencephalography imaging to examine age-related changes of resting-state whole-brain neurophysiology in a well-characterized cohort of cognitively unimpaired individuals (n = 70; age range 52-87 years). We quantified spatial patterns of age-related changes in band-limited spectral power within delta-theta (2-7 Hz), alpha (8-12 Hz) and beta (13-30 Hz) bands and the spectral aperiodic slope (15-50 Hz), and examined how spectral changes are associated with cognitive abilities in healthy ageing. In a subset of individuals (n = 40) who were evaluated with a uniform battery of cognitive tests, using a partial least square regression approach, we examined the associations between age-related spectral changes and cognitive performance. We found that, with advancing age, delta-theta and beta spectral power reduces, while alpha spectral power increases. A periodic slope also showed reductions with ageing. Better cognitive scores were positively correlated with delta-theta reductions and alpha power increases associated with ageing, suggesting that these may represent compensatory neural mechanisms. Beta power reductions and spectral aperiodic slope changes, in contrast, correlated negatively with higher cognitive scores, suggesting that these may represent compromised neural mechanisms of ageing. Our findings highlighted that the neurophysiological changes that occur during later decades of life were distinct from the previously known lifespan changes. This study demonstrates the trajectories of neurophysiological changes in cognitive ageing explicitly relating to conserved and impaired neural mechanisms with important implications for identifying specific spectral changes in neurodegenerative processes in the context of ageing.
Keywords: aperiodic slope; compromised and compensatory brain changes; healthy ageing; magnetoencephalography; neural oscillations.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
K.G.R., K.K., K.C., K.P.R., F.S., K.V., B.L.M., G.D.R., J.H.K. and K.P.R. declare no competing interests relevant to this work. S.S.N. serves as a founding board member in Hippoclinic Inc. and as a private consultant to MEGIN Inc. and is the PI for an industry contract from Ricoh MEG USA. B.L.M. has the following disclosures: serves as Medical Director for the John Douglas French Foundation; Scientific Director for the Tau Consortium; Director/Medical Advisory Board of the Larry L. Hillblom Foundation; and Scientific Advisory Board Member for the National Institute for Health Research Cambridge Biomedical Research Centre and its subunit, the Biomedical Research Unit in Dementia, UK. J.C.R.-M. is a site PI for clinical trials sponsored by Eli Lilly and Eisai and is a consultant for Roon.
Figures




References
-
- Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev. 2004;3(4):369–382. - PubMed
-
- Pearl PL, Beal JC, Eisermann M, et al. Normal EEG in wakefullness and sleep: Preterm; infant; adolescent. In: Schomer D, Lopes da Silva F, eds. Niedermeyer's electroencephalography: Basic principles, clinical applications, and related fields. 7th ed. Oxford University Press; 2018:167–202: chap 7.
-
- Buzsaki G. Rhythms of the brain. Oxford University press; 2011.
Grants and funding
LinkOut - more resources
Full Text Sources
