Photoelectron circular dichroism of aqueous-phase alanine
- PMID: 40255964
- PMCID: PMC12004263
- DOI: 10.1039/d5sc00167f
Photoelectron circular dichroism of aqueous-phase alanine
Abstract
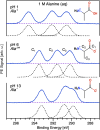
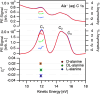
Amino acids and other small chiral molecules play key roles in biochemistry. However, in order to understand how these molecules behave in vivo, it is necessary to study them under aqueous-phase conditions. Photoelectron circular dichroism (PECD) has emerged as an extremely sensitive probe of chiral molecules, but its suitability for application to aqueous solutions had not yet been proven. Here, we report on our PECD measurements of aqueous-phase alanine, the simplest chiral amino acid. We demonstrate that the PECD response of alanine in water is different for each of alanine's carbon atoms, and is sensitive to molecular structure changes (protonation states) related to the solution pH. For C 1s photoionization of alanine's carboxylic acid group, we report PECD of comparable magnitude to that observed in valence-band photoelectron spectroscopy of gas-phase alanine. We identify key differences between PECD experiments from liquids and gases, discuss how PECD may provide information regarding solution-specific phenomena - for example the nature and chirality of the solvation shell surrounding chiral molecules in water - and highlight liquid-phase PECD as a powerful new tool for the study of aqueous-phase chiral molecules of biological relevance.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


Similar articles
-
Detecting chirality in molecules by imaging photoelectron circular dichroism.Phys Chem Chem Phys. 2014 Jan 21;16(3):856-71. doi: 10.1039/c3cp53741b. Epub 2013 Dec 4. Phys Chem Chem Phys. 2014. PMID: 24305955
-
Observation of Photoelectron Circular Dichroism Using a Nanosecond Laser.Chemphyschem. 2019 Jun 4;20(11):1416-1419. doi: 10.1002/cphc.201900289. Epub 2019 May 9. Chemphyschem. 2019. PMID: 30972931
-
Valence-shell photoelectron circular dichroism of ruthenium(III)-tris-(acetylacetonato) gas-phase enantiomers.Phys Chem Chem Phys. 2021 Nov 3;23(42):24140-24153. doi: 10.1039/d1cp02921e. Phys Chem Chem Phys. 2021. PMID: 34666343
-
Two decades of imaging photoelectron circular dichroism: from first principles to future perspectives.Phys Chem Chem Phys. 2025 Feb 6;27(6):2888-2907. doi: 10.1039/d4cp03770g. Phys Chem Chem Phys. 2025. PMID: 39835524 Review.
-
The Clusters-in-a-Liquid Approach for Solvation: New Insights from the Conformer Specific Gas Phase Spectroscopy and Vibrational Optical Activity Spectroscopy.Front Chem. 2016 Feb 25;4:9. doi: 10.3389/fchem.2016.00009. eCollection 2016. Front Chem. 2016. PMID: 26942177 Free PMC article. Review.
Cited by
-
Low-Energy Photoelectron Spectroscopy and Scattering from Aqueous Solutions and the Role of Solute Surface Activity.J Am Chem Soc. 2025 Jun 11;147(23):19868-19877. doi: 10.1021/jacs.5c04263. Epub 2025 Jun 2. J Am Chem Soc. 2025. PMID: 40454638 Free PMC article.
References
-
- Berova N. Di Bari L. Pescitelli G. Application of Electronic Circular Dichroism in Configurational and Conformational Analysis of Organic Compounds. Chem. Soc. Rev. 2007;36:914–931. - PubMed
-
- Nishino H. Kosaka A. Hembury G. A. Matsushima K. Inoue Y. The pH dependence of the anisotropy factors of essential amino acids. J. Chem. Soc., Perkin Trans. 2. 2002:582–590.
-
- Ritchie B. Theory of the angular distribution of photoelectrons ejected from optically active molecules and molecular negative ions. Phys. Rev. A. 1976;13:1411.

