Biotransformation and biological fate of magnetic iron oxide nanoparticles for biomedical research and clinical applications
- PMID: 40255989
- PMCID: PMC12004083
- DOI: 10.1039/d5na00195a
Biotransformation and biological fate of magnetic iron oxide nanoparticles for biomedical research and clinical applications
Abstract
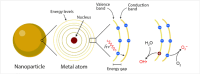
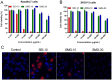
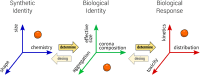
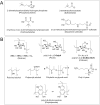
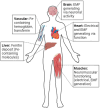
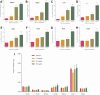
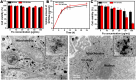
Safe implementation of nanotechnology-based products in biomedical applications necessitates an extensive understanding of the (bio)transformations that nanoparticles undergo in living organisms. The long-term fate in the body is a crucial consideration because it governs potential risks for human health. To accurately predict the life cycle of nanoparticles, their fate after administration into the body-including their (bio)transformations, persistence, and biodegradation-needs to be thoroughly evaluated. Magnetic iron oxide nanoparticles (MIONPs) can enter the body through various routes, including inhalation, ingestion, dermal absorption, and injection. Microscale and nanoscale studies are performed to observe nanomaterial biotransformations and their effect on clinically relevant properties. Researchers are utilizing high-resolution TEM for nanoscale monitoring of the nanoparticles while microscale follow-up approaches comprise quantification tools at the whole organism level and the molecular level. Nanoparticle-cell interactions, including cellular uptake and intracellular trafficking, are key to understanding nanoparticle accumulation in cells and organs. Prolonged accumulation may induce cell stress and nanoparticle toxicity, often mediated through oxidative stress and inflammation. In this review article, the journey of nanoparticles in the body is depicted and their biotransformations and final fate are discussed. Immunohistochemical techniques are particularly valuable in tracking nanoparticle distribution within tissues and assessing their impact at the cellular level. A thorough description of a wide range of characterization techniques is provided to unveil the fate and biotransformations of clinically relevant nanoparticles and to assist in their design for successful biomedical applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Advancing Nanomedicine Through Electron Microscopy: Insights Into Nanoparticle Cellular Interactions and Biomedical Applications.Int J Nanomedicine. 2025 Mar 8;20:2847-2878. doi: 10.2147/IJN.S500978. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40078651 Free PMC article. Review.
-
Long term biotransformation and toxicity of dimercaptosuccinic acid-coated magnetic nanoparticles support their use in biomedical applications.J Control Release. 2013 Oct 28;171(2):225-33. doi: 10.1016/j.jconrel.2013.07.019. Epub 2013 Jul 29. J Control Release. 2013. PMID: 23906866
-
[Life cycle of magnetic nanoparticles in the organism].Biol Aujourdhui. 2014;208(2):177-90. doi: 10.1051/jbio/2014021. Epub 2014 Sep 8. Biol Aujourdhui. 2014. PMID: 25190577 Review. French.
-
Bio-identity and fate of albumin-coated SPIONs evaluated in cells and by the C. elegans model.Acta Biomater. 2016 Oct 1;43:348-357. doi: 10.1016/j.actbio.2016.07.024. Epub 2016 Jul 15. Acta Biomater. 2016. PMID: 27427227
-
Comparative in vitro cytotoxicity study on uncoated magnetic nanoparticles: effects on cell viability, cell morphology, and cellular uptake.J Nanosci Nanotechnol. 2012 Dec;12(12):9010-7. doi: 10.1166/jnn.2012.6755. J Nanosci Nanotechnol. 2012. PMID: 23447952
Cited by
-
Green-synthesized metal nanoparticles: a promising approach for accelerated wound healing.Front Bioeng Biotechnol. 2025 Jul 16;13:1637589. doi: 10.3389/fbioe.2025.1637589. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40741533 Free PMC article. Review.
References
-
- Arms L. Smith D. W. Flynn J. Palmer W. Martin A. Woldu A. Hua S. Advantages and Limitations of Current Techniques for Analyzing the Biodistribution of Nanoparticles. Front. Pharmacol. 2018;9:802. doi: 10.3389/fphar.2018.00802. https://dx.doi.org/10.3389/fphar.2018.00802 - DOI - DOI - PMC - PubMed
-
- Gendelman H. Li T. Boska M. McMillan J. Puligujja P. Zhang G. Bronich T. Liu X. Edagwa B. Magnetic resonance imaging of folic acid-coated magnetite nanoparticles reflects tissue biodistribution of long-acting antiretroviral therapy. Int. J. Nanomed. 2015:3779–3790. doi: 10.2147/IJN.S83279. https://dx.doi.org/10.2147/IJN.S83279 - DOI - DOI - PMC - PubMed
-
- Shakeri-Zadeh A. Bulte J. W. M. Imaging-guided precision hyperthermia with magnetic nanoparticles. Nat. Rev. Bioeng. 2024:245–260. doi: 10.1038/s44222-024-00257-3. https://dx.doi.org/10.1038/s44222-024-00257-3 - DOI - DOI - PMC - PubMed
-
- Rümenapp C. Gleich B. Haase A. Magnetic Nanoparticles in Magnetic Resonance Imaging and Diagnostics. Pharm. Res. 2012;29:1165–1179. doi: 10.1007/s11095-012-0711-y. https://dx.doi.org/10.1007/s11095-012-0711-y - DOI - DOI - PubMed
-
- Bin Na H. Song I. C. Hyeon T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009;21:2133–2148. doi: 10.1002/adma.200802366. https://dx.doi.org/10.1002/adma.200802366 - DOI - DOI
Publication types
LinkOut - more resources
Full Text Sources

