Targeting ceramide-induced microglial pyroptosis: Icariin is a promising therapy for Alzheimer's disease
- PMID: 40256246
- PMCID: PMC12008632
- DOI: 10.1016/j.jpha.2024.101106
Targeting ceramide-induced microglial pyroptosis: Icariin is a promising therapy for Alzheimer's disease
Abstract
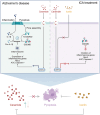
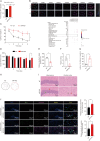
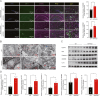
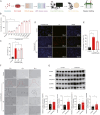
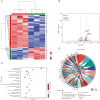
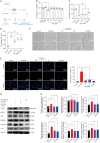
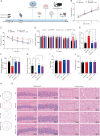
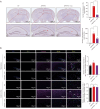
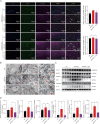
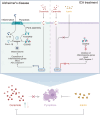
Alzheimer's disease (AD), a progressive dementia, is one of the most common neurodegenerative diseases. Clinical trial results of amyloid-β (Aβ) and tau regulators based on the pretext of straightforward amyloid and tau immunotherapy were disappointing. There are currently no effective strategies for slowing the progression of AD. Herein, we spotlight the dysregulation of lipid metabolism, particularly the elevation of ceramides (Cers), as a critical yet underexplored facet of AD pathogenesis. Our study delineates the role of Cers in promoting microglial pyroptosis, a form of programmed cell death distinct from apoptosis and necroptosis, characterized by cellular swelling, and membrane rupture mediated by the NLRP3 inflammasome pathway. Utilizing both in vivo experiments with amyloid precursor protein (APP)/presenilin 1 (PS1) transgenic mice and in vitro assays with BV-2 microglial cells, we investigate the activation of microglial pyroptosis by Cers and its inhibition by icariin (ICA), a flavonoid with known antioxidant and anti-inflammatory properties. Our findings reveal a significant increase in Cers levels and pyroptosis markers (NOD-like receptor family, pyrin domain containing 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase recruitment domain, caspase-1, gasdermin D (gasdermin D (GSDMD)), and interleukin-18 (IL-18)) in the brains of AD model mice, indicating a direct involvement of Cers in AD pathology through the induction of microglial pyroptosis. Conversely, ICA treatment effectively reduces these pyroptotic markers and Cer levels, thereby attenuating microglial pyroptosis and suggesting a novel therapeutic mechanism of action against AD. This study not only advances our understanding of the pathogenic role of Cers in AD but also introduces ICA as a promising candidate for AD therapy, capable of mitigating neuroinflammation and pyroptosis through the cyclooxygenase-2 (COX-2)-NLRP3 inflammasome-gasdermin D (GSDMD) axis. Our results pave the way for further exploration of Cer metabolism disorders in neurodegenerative diseases and highlight the therapeutic potential of targeting microglial pyroptosis in AD.
Keywords: Alzheimer's disease; COX2; Ceramides; Icariin; Microglia pyroptosis; NLRP3 inflammasome.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Effects of amyloid β (Aβ)42 and Gasdermin D on the progression of Alzheimer's disease in vitro and in vivo through the regulation of astrocyte pyroptosis.Aging (Albany NY). 2023 Nov 2;15(21):12209-12224. doi: 10.18632/aging.205174. Epub 2023 Nov 2. Aging (Albany NY). 2023. PMID: 37921870 Free PMC article.
-
Pyroptosis in Alzheimer's disease: cell type-specific activation in microglia, astrocytes and neurons.Acta Neuropathol. 2023 Feb;145(2):175-195. doi: 10.1007/s00401-022-02528-y. Epub 2022 Dec 9. Acta Neuropathol. 2023. PMID: 36481964
-
Copper Overload Promotes β-amyloid Induced NLRP3/Caspase-1/GSDMD-Mediated Pyroptosis in Alzheimer's Disease.J Integr Neurosci. 2024 Oct 21;23(10):194. doi: 10.31083/j.jin2310194. J Integr Neurosci. 2024. PMID: 39473154
-
Mechanisms of NLRP3 Inflammasome Activation: Its Role in the Treatment of Alzheimer's Disease.Neurochem Res. 2020 Nov;45(11):2560-2572. doi: 10.1007/s11064-020-03121-z. Epub 2020 Sep 14. Neurochem Res. 2020. PMID: 32929691 Review.
-
A novel strategy for bioactive natural products targeting NLRP3 inflammasome in Alzheimer's disease.Front Pharmacol. 2023 Jan 9;13:1077222. doi: 10.3389/fphar.2022.1077222. eCollection 2022. Front Pharmacol. 2023. PMID: 36699095 Free PMC article. Review.
References
-
- Jia J., Ning Y., Chen M., et al. Biomarker changes during 20 years preceding Alzheimer's disease. N. Engl. J. Med. 2024;390:712–722. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

