A Non-randomized, Open-label, Multicenter Study to Evaluate the Safety and Tolerability of 10% Aminolevulinic Acid Gel in the Expanded Field-directed Treatment of Actinic Keratosis on the Face and Scalp with Red Light Photodynamic Therapy
- PMID: 40256341
- PMCID: PMC12007652
A Non-randomized, Open-label, Multicenter Study to Evaluate the Safety and Tolerability of 10% Aminolevulinic Acid Gel in the Expanded Field-directed Treatment of Actinic Keratosis on the Face and Scalp with Red Light Photodynamic Therapy
Abstract
Objective: Dermatologists regularly encounter patients having expanded fields with numerous actinic keratosis (AK) lesions on the face and scalp. Field-directed red light photodynamic therapy (PDT) is a well-established treatment, yet published data on the safety of PDT on large areas is scarce. We aimed to evaluate the safety and tolerability of red light PDT in treating expanded AK fields on the face and scalp.
Methods: This was a non-randomized, open-label, multicenter study. After lesion preparation, 6g of 10% aminolevulinic acid (ALA) gel were applied to the treatment field (60 cm2) and incubated for three hours under a light-blocking, occlusive dressing before 10-minute illumination with a red light lamp (~635nm, 37 J/cm2). Safety and tolerability were assessed throughout the study.
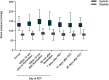
Results: All participants (n=100) had adverse reactions. No previously unknown effects, serious adverse events, or deaths were reported. The most frequent application site reactions were pain/burning (96.0%), exfoliation (87.0%), and erythema (86.0%). Most treatment-emergent adverse events were of mild to moderate severity and lasted slightly longer compared to those experienced after treatment of smaller areas. The mean maximum pain during PDT was 7.4±2.1 on an 11-point numeric rating scale. A transient increase in blood pressure on the day that PDT was performed was not clinically significant.
Limitations: Although the allowed use of pain-reducing measures might have influenced evaluation of pain, it reflects how the procedure is managed in current practice.
Concklusion: PDT with 10% ALA gel and red light illumination on an expanded treatment field was generally well tolerated.
Keywords: 10% ALA gel; BF-200 ALA; Photodynamic therapy; RhodoLED; actinic keratoses; actinic keratosis; aminolevulinic acid; clinical research; red light; safety.
Copyright © 2025. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
DISCLOSURES: Dr. Cohen has served as a clinical research principal investigator as well as a consultant for Biofrontera. Dr. Couvillion is an investigator for AbbVie, Alumis, AnaptysBio, ArcutisBiotherapeutics, Biofrontera Bioscience GmbH, Cara Therapeutics, Castle Biosciences, Concert Pharmaceuticals, Dr. Reddy’s Laboratories, Eirion Therapeutics, Incyte, Janssen Biotech, Nielsen Bioscience Inc., Ortho Dermatologics, Timber Pharmaceuticals, TrialSpark, Inc., VeraDermics Inc., and Verrica Pharmaceuticals, is an investigator and speaker for Eli Lilly and Pfizer Inc., is a speaker for Revance therapeutics Inc., and consultant for Allergan Aesthetics and Dermavant Sciences Inc. Dr. Zeitouni is a consultant and investigator for Biofrontera Biosciences GmbH. Dr. Ibrahim is an investigator for Biofrontera Bioscience GmbH. Dr. Hanke is an investigator for AbbVie, Biofrontera Bioscience GmbH and Castle Biosciences, Inc., and received honoraria as a speaker for Sanofi/Regeneron and Sun Pharmaceutical Industries Ltd. Dr. Lain declares financial relationships with AbbVie, Aclaris Therapeutics, Allergan Inc, Almirall, Arcutis, ASLAN Pharmaceuticals, AstraZeneca, Athenix, Biopelle Inc, BioPharmX, Biorasi LLC, Bristol-Myers Squibb, Bricknell Biotech Inc., Cassiopea S.p.a., Celgene, Cellceutics, ChemoCentryx, Cutanea Life Sciences, Cutera Inc, Dermira, Dermavant Sciences Inc., Dow Pharmaceutical Sciences, Dr. Reddy’s Laboratory, Eli Lilly, Gage Development Company LLC, Galderma, Hovione, Incyte, Kadmon, La Roche-Posay, LEO Pharma, Medimmune, Menlo Therapeutics, Neothetics, Nielsen Holdings N.V, Novartis, Ortho Dermatologics, Pfizer, Promius Pharma LLC, Sebacia Inc., Sienna Labs Inc., SkinCeuticals LLC, Sol-Gel Technologies, UCB, Valeant Pharmaceuticals, and Verrica Pharmaceuticals. Drs. Zogel and Schmitz were employees of Biofrontera Bioscience GmbH at the time the study was conducted. Dr. Zeuner was an employee of Biofrontera Bioscience GmbH at the time the study results were accessible, and the manuscript was prepared. Dr. Schlesinger is a consultant and investigator for AbbVie, Almirall, Biofrontera Bioscience GmbH, Galderma and a consultant for SUN Pharma. Drs. Tu and Jazayeri have no conflicts of interest to report. Parts of the data were presented at the 44th Annual Fall Clinical Dermatology Conference® (October 2024).
Figures


References
-
- Warino L, Tusa M, Camacho F et al. Frequency and cost of actinic keratosis treatment. Dermatol Surg. 2006;32(8):1045–1049. - PubMed
-
- Lim HW, Collins SAB, Resneck JS, Jr et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958–972.e2. - PubMed
-
- Heppt MV, Leiter U, Steeb T et al. S3 guideline "actinic keratosis and cutaneous squamous cell carcinoma"- update 2023, part 1: Treatment of actinic keratosis, actinic cheilitis, cutaneous squamous cell carcinoma in situ (Bowen's disease), occupational disease and structures of care. J Dtsch Dermatol Ges. 2023;21(10):1249–1262. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
