Advances in the mechanism and therapies of achondroplasia
- PMID: 40256430
- PMCID: PMC12008630
- DOI: 10.1016/j.gendis.2024.101436
Advances in the mechanism and therapies of achondroplasia
Abstract
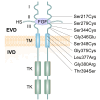
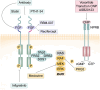
Achondroplasia (ACH), is the prevailing type of genetic dwarfism in humans, caused by mutations in fibroblast growth factor receptor 3 (FGFR3) that are inherited in an autosomal dominant manner. FGFR3 is mainly expressed in condensed mesenchyme, chondrocytes, and mature osteoblasts and osteoclasts, in which it regulates the formation, development, growth, and remodeling of the skeletal system. Mutations in FGFR3 causing ACH result in enhanced FGFR3 signaling through combined mechanisms including enhancing FGF dimerization and tyrosine kinase activity and stabilizing FGF receptors. In ACH, suppression of the proliferation and maturation of chondrocytes in the growth plate leads to a notable reduction in growth plate size, trabecular bone volume, and bone elongation through a profound enhancement of FGFR3 signaling. This review aims to comprehensively outline the cellular and molecular mechanisms contributing to the pathological process of ACH and its potential therapeutic interventions.
Keywords: Achondroplasia; FGFR3; Mechanisms; Skeleton development; Therapeutic interventions.
© 2025 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltdé.
Conflict of interest statement
The authors declared no conflict of interests.
Figures
Similar articles
-
Achondroplasia: Development, pathogenesis, and therapy.Dev Dyn. 2017 Apr;246(4):291-309. doi: 10.1002/dvdy.24479. Epub 2017 Mar 2. Dev Dyn. 2017. PMID: 27987249 Free PMC article. Review.
-
An Fgfr3-activating mutation in immature murine osteoblasts affects the appendicular and craniofacial skeleton.Dis Model Mech. 2021 Apr 1;14(4):dmm048272. doi: 10.1242/dmm.048272. Epub 2021 Apr 23. Dis Model Mech. 2021. PMID: 33737326 Free PMC article.
-
Differential regulation of endochondral bone growth and joint development by FGFR1 and FGFR3 tyrosine kinase domains.Development. 2001 Oct;128(19):3867-76. doi: 10.1242/dev.128.19.3867. Development. 2001. PMID: 11585811
-
Gain-of-function mutation in FGFR3 in mice leads to decreased bone mass by affecting both osteoblastogenesis and osteoclastogenesis.Hum Mol Genet. 2010 Apr 1;19(7):1199-210. doi: 10.1093/hmg/ddp590. Epub 2010 Jan 6. Hum Mol Genet. 2010. PMID: 20053668 Free PMC article.
-
C-Type Natriuretic Peptide Analog as Therapy for Achondroplasia.Endocr Dev. 2016;30:98-105. doi: 10.1159/000439334. Epub 2015 Dec 10. Endocr Dev. 2016. PMID: 26684019 Review.
References
-
- Savarirayan R., Ireland P., Irving M., et al. International Consensus Statement on the diagnosis, multidisciplinary management and lifelong care of individuals with achondroplasia. Nat Rev Endocrinol. 2022;18(3):173–189. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

