Piperazine-Based Co(III), Ni(II), Cu(II), and Zn(II) Carbodithioate Complexes as Potential Anticancer Agent
- PMID: 40256499
- PMCID: PMC12004137
- DOI: 10.1021/acsomega.4c06972
Piperazine-Based Co(III), Ni(II), Cu(II), and Zn(II) Carbodithioate Complexes as Potential Anticancer Agent
Abstract
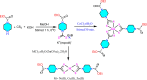
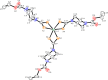
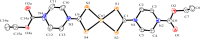
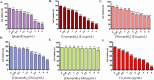
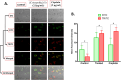
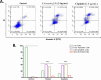
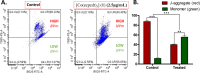
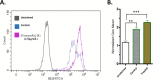
The development of facile and cost-effective anticancer metallodrugs possessing minimal side effects is urgently needed. Piperazine-containing anticancer drugs are already available on the market. A piperazine-based potassium 4-(ethoxycarbonyl)piperazine-1-carbodithioate [pecpcdt] (L) ligand and its metal complexes [Co(ecpcdt)3] (1), [Ni(ecpcdt)2] (2), [Cu(ecpcdt)2] (3), and [Zn(ecpcdt)2] (4) were synthesized. These compounds were characterized by different spectroscopic methods and single-crystal X-ray crystallography data. Ni(II) and Cu(II) complexes have distorted square planar geometry, whereas the Co(III) complex has distorted octahedral geometry around the metal ions. Complexes are weakly fluorescent in the solution compared to the free ligand. The complexes were further examined for their in vitro anticancer activities against the primary Dalton's lymphoma (DL) cells along with standard drug cisplatin. The anticancer studies of metal complexes have been performed through various biochemical assays, and the findings thus obtained suggest that they demonstrate an effective anticancer activity. [Co(ecpcdt)3] (1) shows superior cytotoxicity against DL cells than complexes [Cu(ecpcdt)2] (3), [Zn(ecpcdt)2] (4), and cisplatin. The superiority preferences of these complexes follows [Co(ecpcdt)3] (1) > [pecpcdt] > [Cu(ecpcdt)2] (3) > [Ni(ecpcdt)2] (2) > [Zn(ecpcdt)2] (4). Further assays were performed on a cobalt(III) complex having the highest efficacy to gain insights into the mechanism of cell death and showed that reduced mitochondrial membrane potential and increased mitochondrial ROS production, highlighting mitochondrial-dependent apoptosis as the major mechanism for tumor cell death. On the other hand, the viability of normal splenocytes was minimally affected by the [Co(ecpcdt)3] (1) treatment.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Synthesis, characterization, and ligand exchange reactivity of a series of first row divalent metal 3-hydroxyflavonolate complexes.Inorg Chem. 2010 Jan 4;49(1):82-96. doi: 10.1021/ic901405h. Inorg Chem. 2010. PMID: 19954165
-
Syntheses, structures, and magnetic properties of acetato- and diphenolato-bridged 3d-4f binuclear complexes [M(3-MeOsaltn)(MeOH)x(ac)Ln(hfac)2] (M = Zn(II), Cu(II), Ni(II), Co(II); Ln = La(III), Gd(III), Tb(III), Dy(III); 3-MeOsaltn = N,N'-bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato; ac = acetato; hfac = hexafluoroacetylacetonato; x = 0 or 1).Inorg Chem. 2013 May 20;52(10):6160-78. doi: 10.1021/ic400594u. Epub 2013 May 6. Inorg Chem. 2013. PMID: 23646986
-
Synthesis, characterization and DNA binding/cleavage, protein binding and cytotoxicity studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes of aminonaphthoquinone.J Photochem Photobiol B. 2017 Mar;168:165-174. doi: 10.1016/j.jphotobiol.2017.02.010. Epub 2017 Feb 16. J Photochem Photobiol B. 2017. PMID: 28231533
-
8-Hydroxyquinoline derived p-halo N4-phenyl substituted thiosemicarbazones: Crystal structures, spectral characterization and in vitro cytotoxic studies of their Co(III), Ni(II) and Cu(II) complexes.Bioorg Chem. 2021 Jul;112:104962. doi: 10.1016/j.bioorg.2021.104962. Epub 2021 May 5. Bioorg Chem. 2021. PMID: 33992968
-
Flavonolate complexes of M(II) (M = Mn, Fe, Co, Ni, Cu, and Zn). Structural and functional models for the ES (enzyme-substrate) complex of quercetin 2,3-dioxygenase.Inorg Chem. 2013 Oct 7;52(19):10936-48. doi: 10.1021/ic400972k. Epub 2013 Sep 17. Inorg Chem. 2013. PMID: 24044415
Cited by
-
Antitumor potential of ivermectin against T-cell lymphoma-bearing hosts.Med Oncol. 2025 Apr 21;42(5):169. doi: 10.1007/s12032-025-02726-0. Med Oncol. 2025. PMID: 40257544
References
-
- Chanu M. T.; Asem S. S. Cancer Disease and Its’ Understanding from the Ancient Knowledge to the Modern Concept. World J. Adv. Res. Rev. 2022, 15 (2), 169–176. 10.30574/wjarr.2022.15.2.0809. - DOI
-
- Chaurasia R.; Pandey S. K.; Singh D. K.; Bharty M. K.; Ganesan V.; Hira S. K.; Manna P. P.; Bharti A.; Butcher R. J. Antiproliferative Activity and Electrochemical Oxygen Evolution by Ni(II) Complexes of N ′-(Aroyl)-Hydrazine Carbodithioates. Dalton Trans. 2021, 50 (40), 14362–14373. 10.1039/D1DT02285G. - DOI - PubMed
LinkOut - more resources
Full Text Sources
