On Levodopa Interactions with Brain Disease Amyloidogenic Proteins at the Nanoscale
- PMID: 40256523
- PMCID: PMC12004170
- DOI: 10.1021/acsomega.5c01028
On Levodopa Interactions with Brain Disease Amyloidogenic Proteins at the Nanoscale
Abstract
The cerebral accumulation of α-synuclein (α-Syn) and amyloid β-1-42 (Aβ-42) proteins is known to play a key role in the pathology of Parkinson's disease (PD). Currently, levodopa (L-dopa) is the first-line dopamine replacement therapy for treating bradykinetic symptoms (i.e., difficulty initiating physical movements), which become visible in PD patients. Using atomic force microscopy, we evidence at nanometer length scales the differential effects of L-dopa on the morphology of α-Syn and Aβ-42 protein fibrils. L-dopa treatment was observed to reduce the length and diameter of both types of protein fibrils, with a stark reduction mainly observed for Aβ-42 fibrils in physiological buffer solution and human cerebrospinal fluid. The insights gained on Aβ-42 fibril disassembly from the label-free nanoscale imaging experiments are substantiated by using atomic-scale molecular dynamics simulations. Our results indicate L-dopa-driven reversal of amyloidogenic protein aggregation, which might provide leads for designing chemical effector-mediated disassembly of insoluble protein aggregates.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


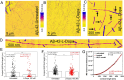
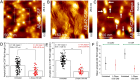

References
-
- Bhattacharya S.; Xu L.; Thompson D. Revisiting the earliest signatures of amyloidogenesis: Roadmaps emerging from computational modeling and experiment. WIREs Comput. Mol. Sci. 2018, 8 (4), e135910.1002/wcms.1359. - DOI
-
- Xu L.; Bhattacharya S.; Thompson D., Predictive Modeling of Neurotoxic α-Synuclein Polymorphs. In Computer Simulations of Aggregation of Proteins and Peptides; Springer US: New York, NY, 2022; 379–399.
LinkOut - more resources
Full Text Sources
Miscellaneous
