Preparative Coupled Enzymatic Synthesis of L-Homophenylalanine and 2-Hydroxy-5-oxoproline with Direct In Situ Product Crystallization and Cyclization
- PMID: 40256536
- PMCID: PMC12004150
- DOI: 10.1021/acsomega.5c00590
Preparative Coupled Enzymatic Synthesis of L-Homophenylalanine and 2-Hydroxy-5-oxoproline with Direct In Situ Product Crystallization and Cyclization
Abstract
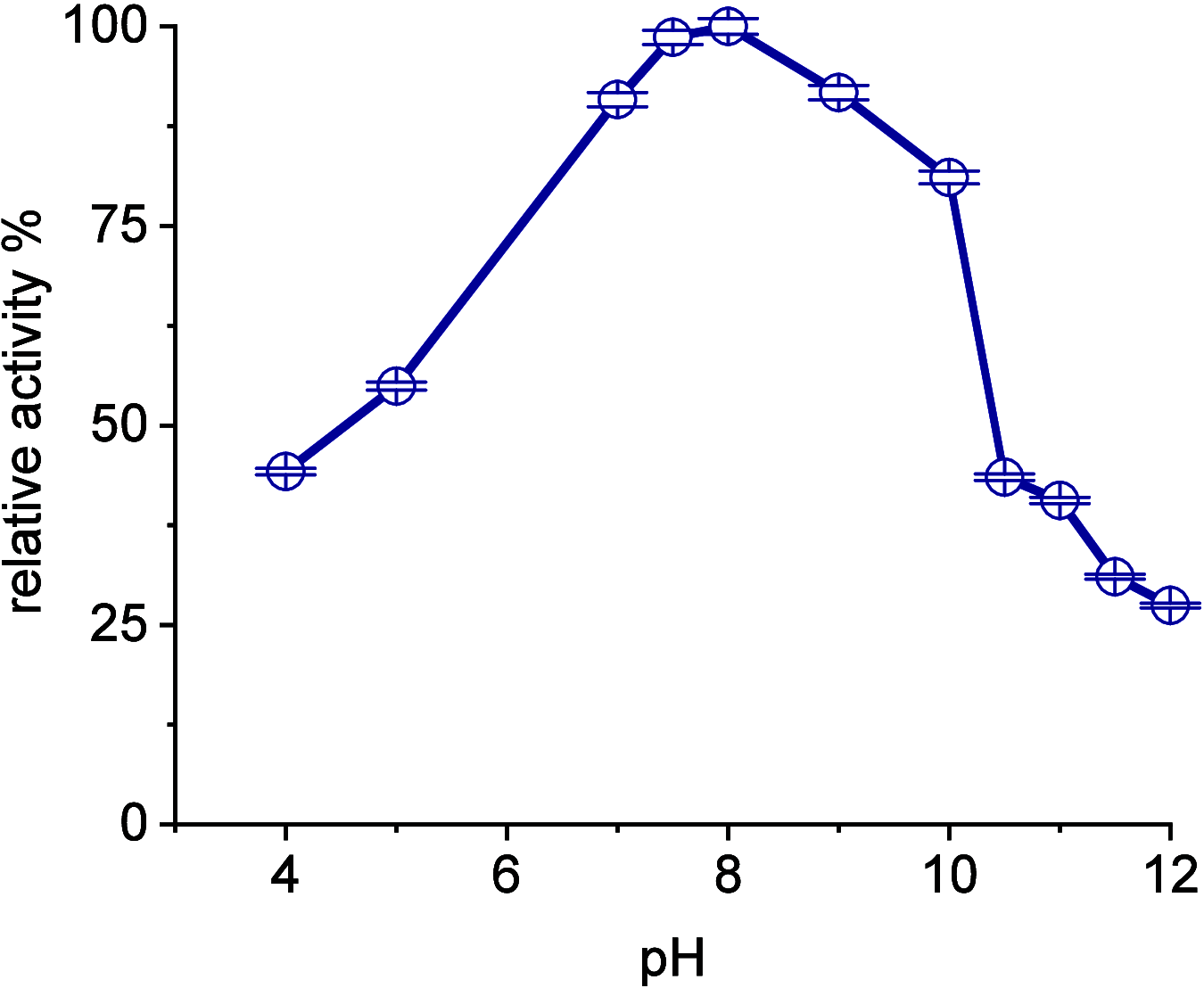
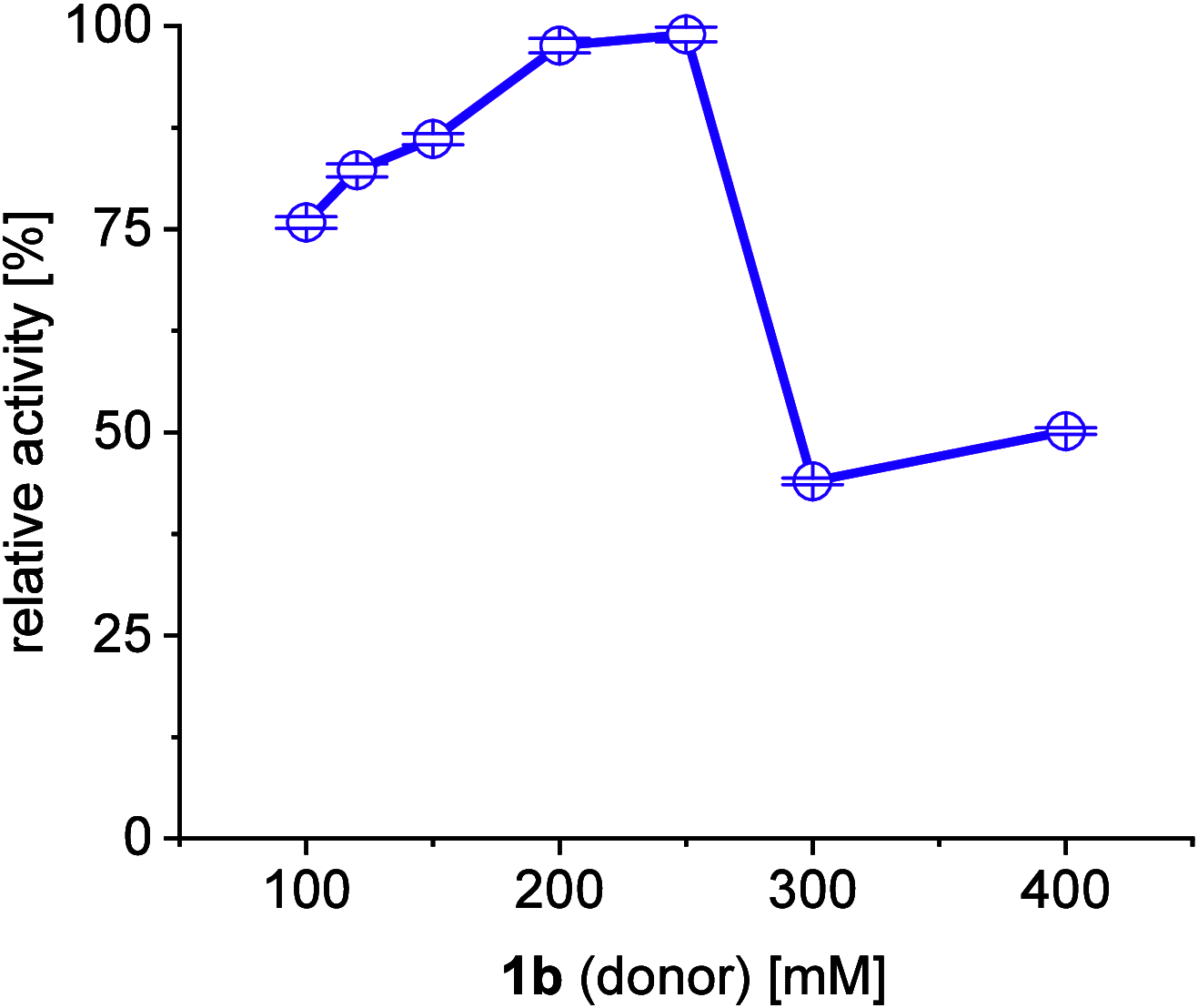
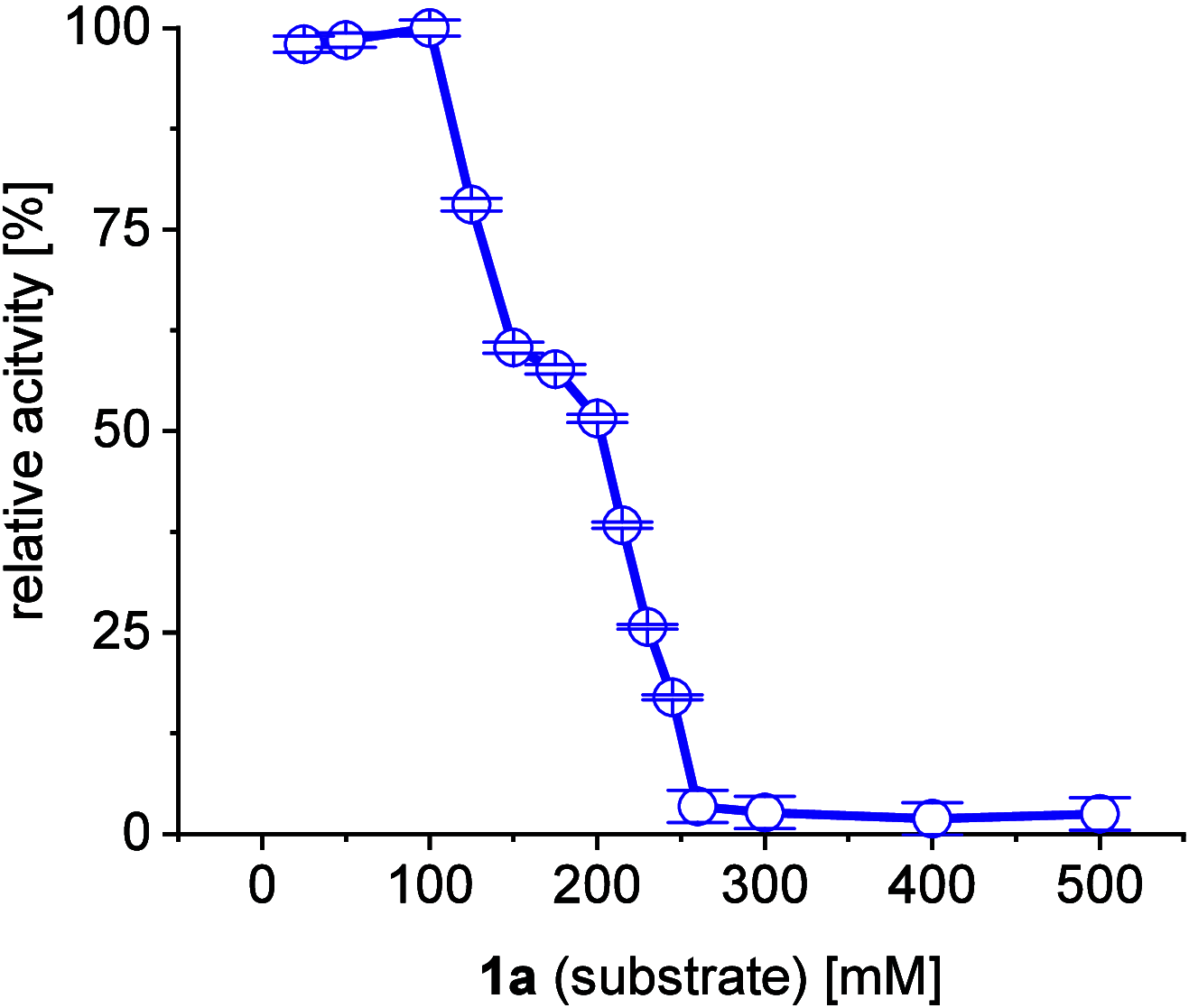
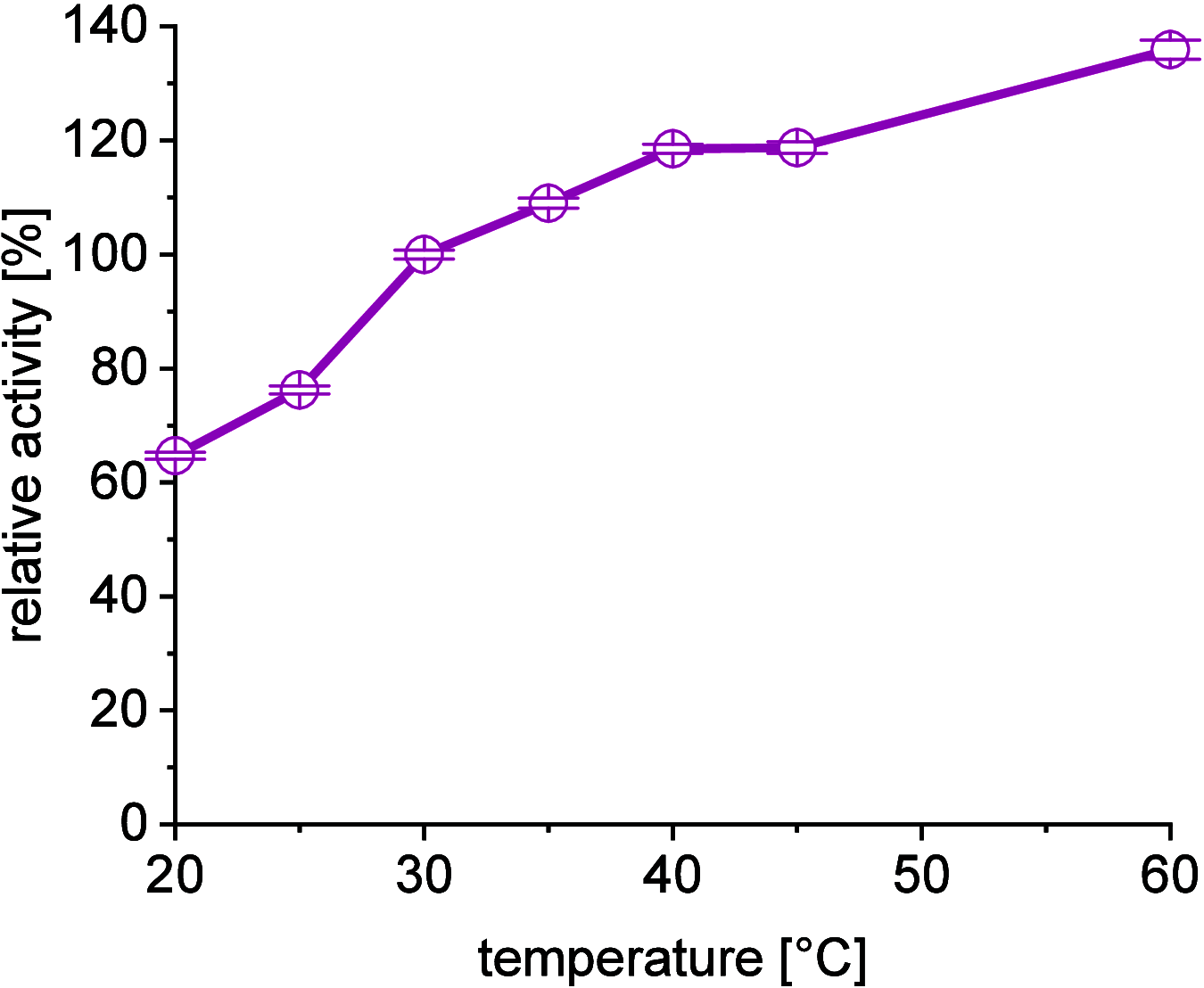
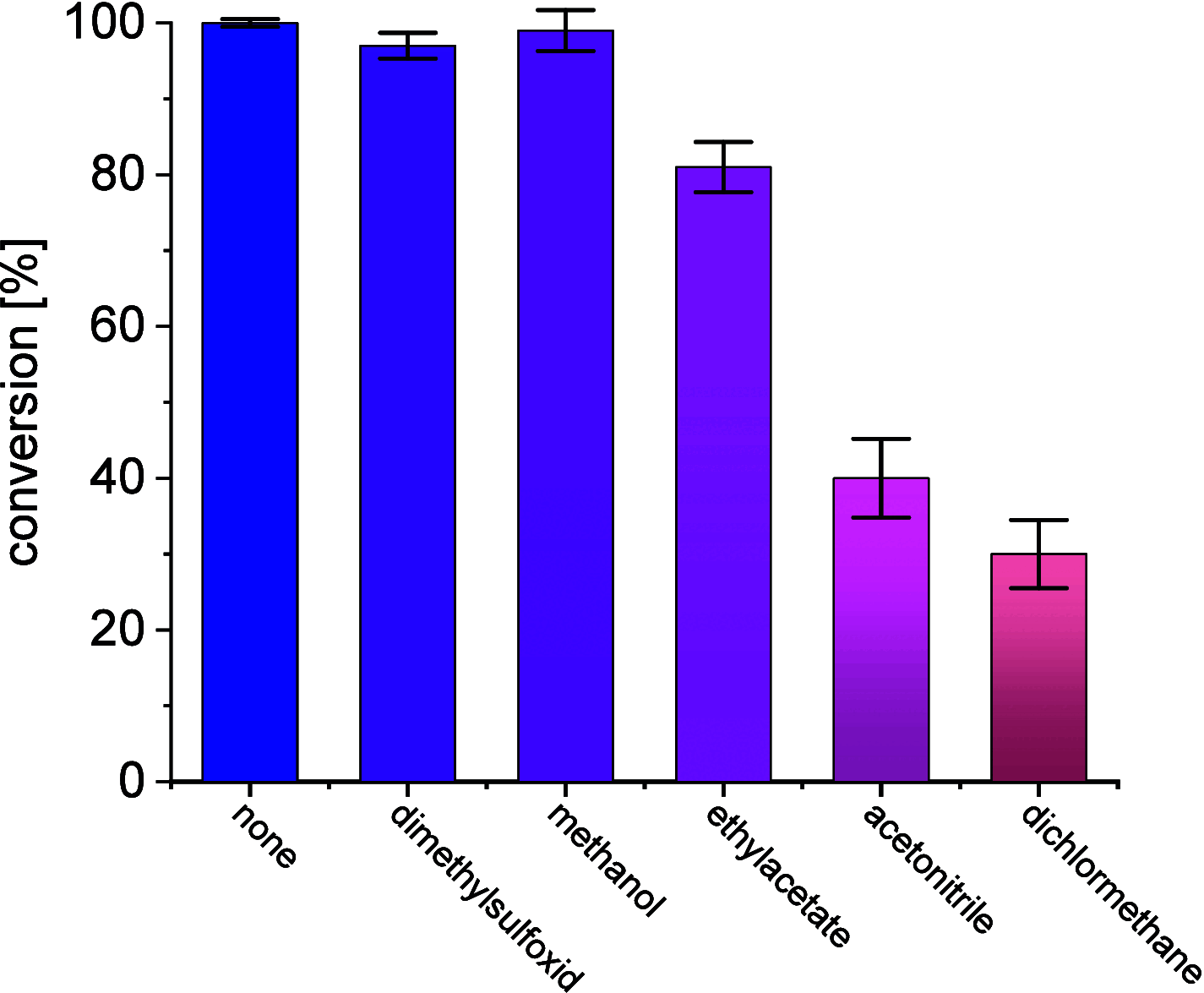
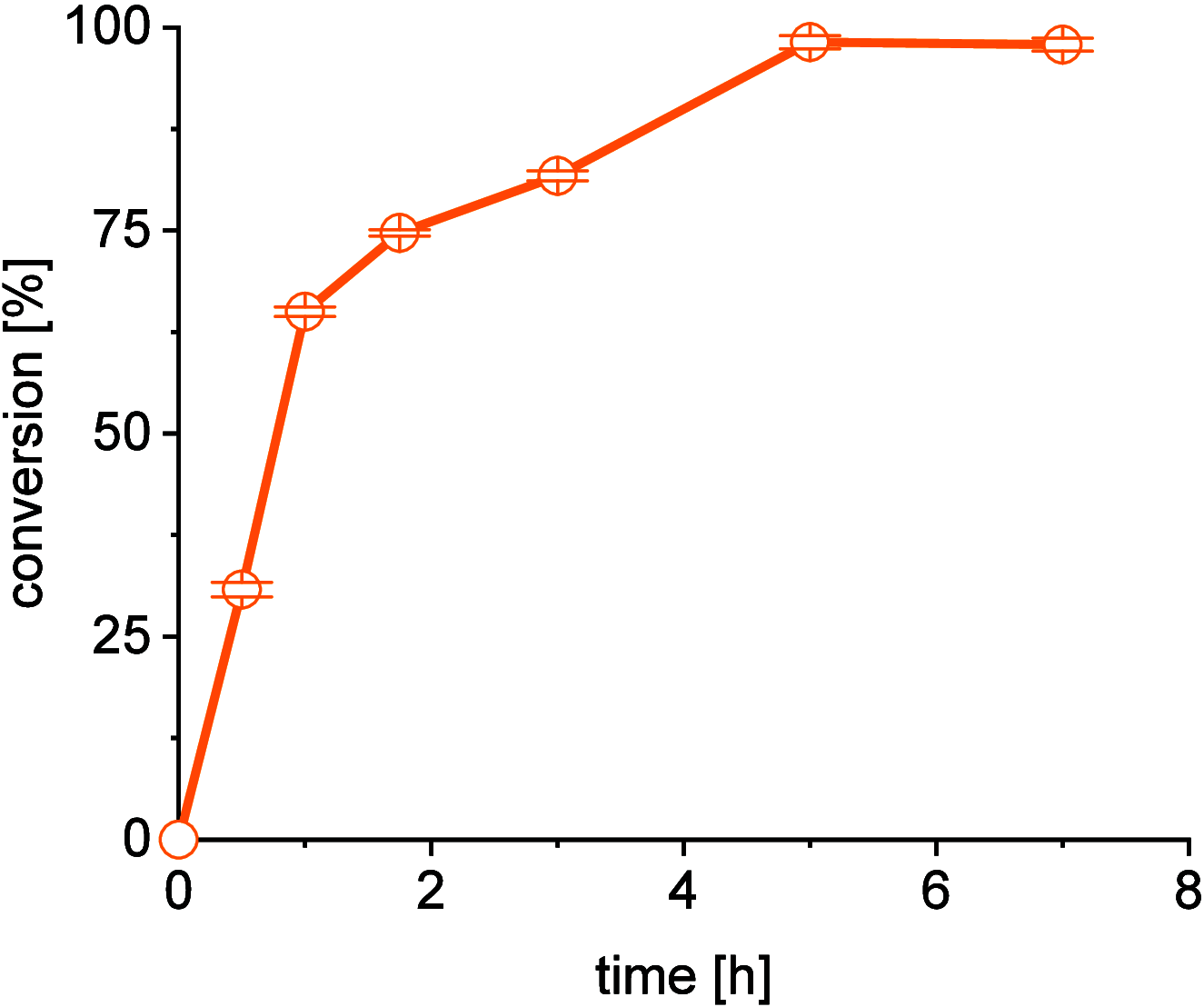
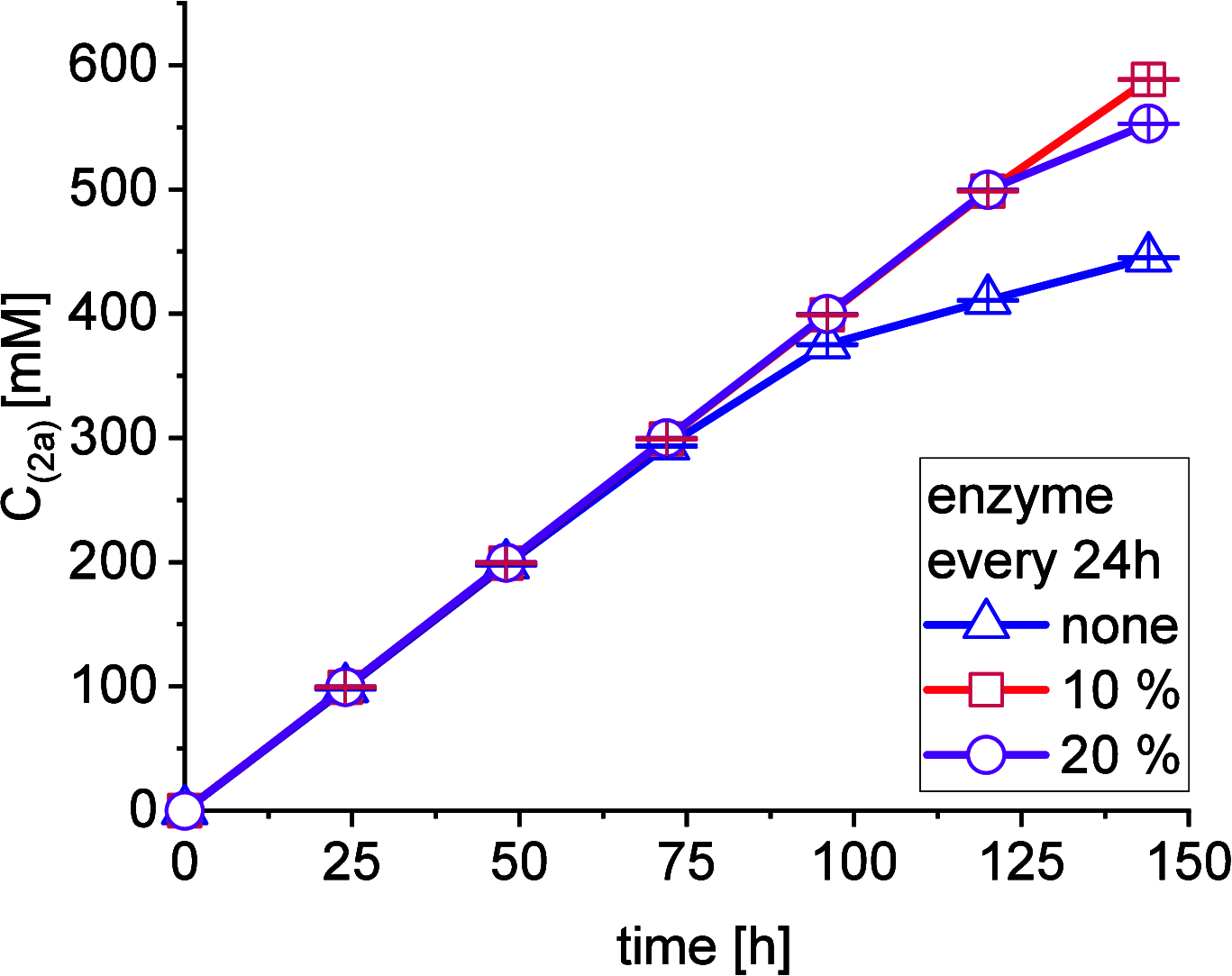
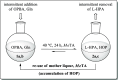
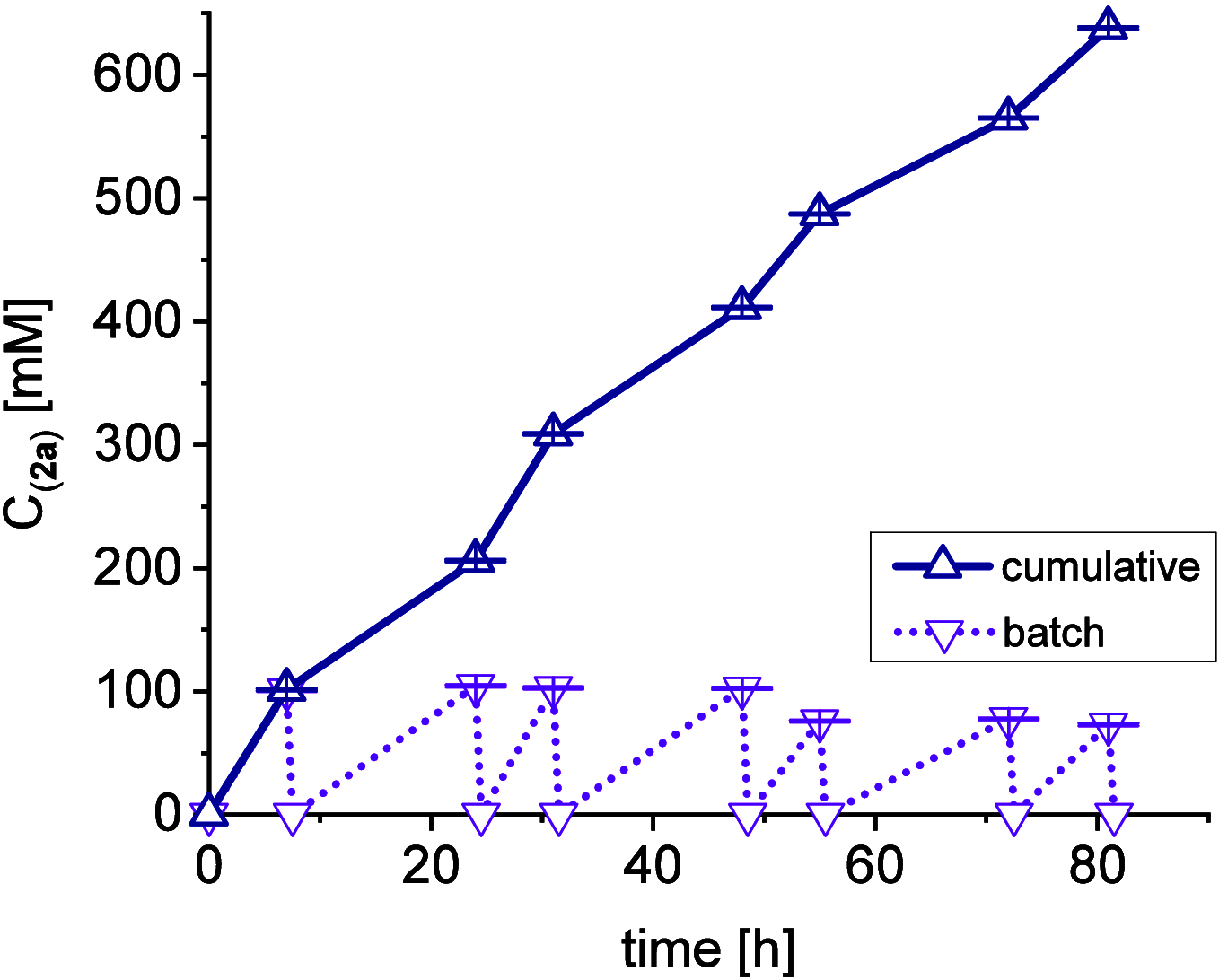
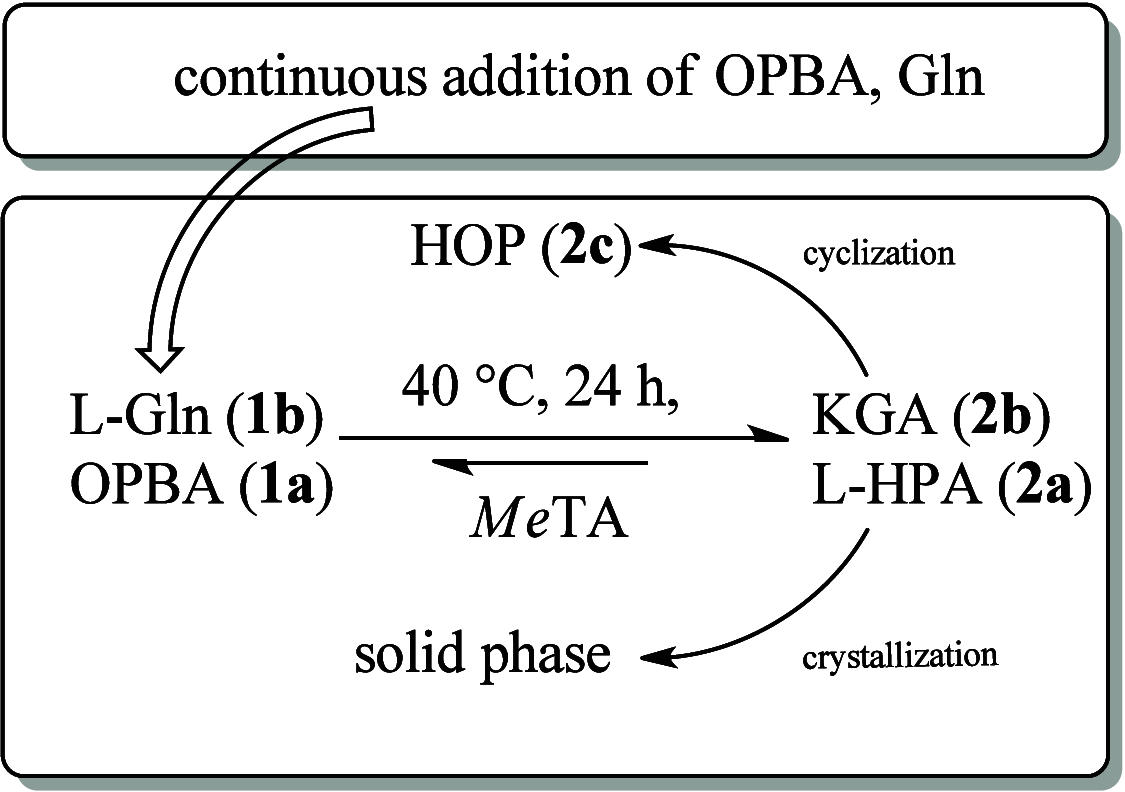
A continuous in situ crystallization concept is presented for the coupled preparative synthesis of L-homophenylalanine and 2-hydroxy-5-oxoproline (a cyclized form of α-ketoglutarate) using the α-transaminase from Megasphaera elsdenii. The process consists of a spontaneous reactive crystallization step of the enantiopure amino acid itself and a parallel spontaneous cyclization of the deaminated cosubstrate in solution. In parallel, these effects significantly improve the overall productivity of the biocatalytic reaction. Batch, repetitive, and fed-batch processes were investigated, and the fed-batch option proved to be the most viable option. The fed-batch process was subsequently used for a coupled synthesis approach at the gram scale. In total, >18 g of chemically pure L-homophenylalanine and >9 g of 2-hydroxy-5-oxoproline were isolated. This optimized process allows for the design of effective transaminase-catalyzed reactions at a preparative scale utilizing standard (fed-)batch-mode crystallizers.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Enhancement of biocatalytic efficiency by increasing substrate loading: enzymatic preparation of L-homophenylalanine.Appl Microbiol Biotechnol. 2013 Oct;97(19):8487-94. doi: 10.1007/s00253-013-5117-1. Epub 2013 Jul 28. Appl Microbiol Biotechnol. 2013. PMID: 23893309
-
Resolution of Racemic Guaifenesin Applying a Coupled Preferential Crystallization-Selective Dissolution Process: Rational Process Development.Cryst Growth Des. 2019 Jun 5;19(6):3148-3157. doi: 10.1021/acs.cgd.8b01660. Epub 2019 May 1. Cryst Growth Des. 2019. PMID: 32952448 Free PMC article.
-
Crystallization Assisted Dynamic Kinetic Resolution for the Synthesis of (R)-β-Methylphenethylamine.Chembiochem. 2024 Aug 19;25(16):e202400203. doi: 10.1002/cbic.202400203. Epub 2024 May 15. Chembiochem. 2024. PMID: 38602845
-
Sustainable biocatalytic synthesis of L-homophenylalanine as pharmaceutical drug precursor.Biotechnol Adv. 2009 May-Jun;27(3):286-96. doi: 10.1016/j.biotechadv.2009.01.003. Epub 2009 Jan 22. Biotechnol Adv. 2009. PMID: 19500550 Review.
-
[Advances in enzymatic production of L-homophenylalanine].Sheng Wu Gong Cheng Xue Bao. 2023 Aug 25;39(8):3111-3124. doi: 10.13345/j.cjb.230052. Sheng Wu Gong Cheng Xue Bao. 2023. PMID: 37622351 Review. Chinese.
References
-
- Belov F.; Mildner A.; Knaus T.; Mutti F. G.; von Langermann J. Crystallization-based downstream processing of ω-transaminase- and amine dehydrogenase-catalyzed reactions. React. Chem. Eng. 2023, 8 (6), 1427–1439. 10.1039/D2RE00496H. - DOI
-
- Neuburger J. E.; Gazizova A.; Tiedemann S.; von Langermann J. Chemoenzymatic Synthesis of Enantiopure Amino Alcohols from Simple Methyl Ketones. Eur. J. Org. Chem. 2023, 26 (34), e20220147110.1002/ejoc.202201471. - DOI
LinkOut - more resources
Full Text Sources
