In Silico and In Vivo Investigation of the Anti-Hyperglycemic Effects of Caffeic Acid
- PMID: 40256540
- PMCID: PMC12004181
- DOI: 10.1021/acsomega.4c11062
In Silico and In Vivo Investigation of the Anti-Hyperglycemic Effects of Caffeic Acid
Abstract
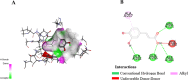
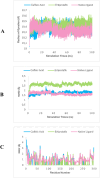
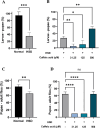
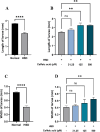
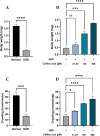
Hyperglycemia, characterized by elevated blood glucose levels, is a major risk factor for diabetes mellitus and its complications. While conventional therapies are effective, they are often associated with side effects and high costs, necessitating alternative strategies. This study evaluates the potential of caffeic acid (CA), a phenolic compound with reported antihyperglycemic properties, using both in silico and in vivo approaches. Molecular docking simulations revealed that CA demonstrates a strong binding affinity to protein tyrosine phosphatase 1B (PTP1B), a critical enzyme in glucose metabolism, with superior interaction profiles compared to the reference drug, ertiprotafib. In the in vivo studies, a Drosophila melanogaster model was used to investigate the effects of CA under hyperglycemic conditions induced by a high-sugar diet. Treatment with CA, particularly at a concentration of 500 μM, significantly reduced hemolymph glucose levels and improved several physiological and behavioral parameters, including survival rates, body size, body weight, and larval movement. Furthermore, gene expression analysis demonstrated that CA modulates key metabolic and stress-related pathways, enhancing glucose homeostasis and reducing metabolic stress. These findings highlight the dual utility of in silico and in vivo methodologies in elucidating the antihyperglycemic potential of CA. The results support the development of CA as a cost-effective and ethically viable therapeutic candidate with implications for diabetes management in resource-limited settings.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
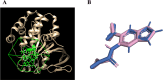

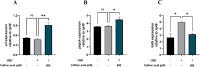
References
-
- Ong K. L.; Stafford L. K.; McLaughlin S. A.; Boyko E. J.; Vollset S. E.; Smith A. E.; Dalton B. E.; Duprey J.; Cruz J. A.; Hagins H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402 (10397), 203–234. 10.1016/s0140-6736(23)01301-6. - DOI - PMC - PubMed
-
- Ansari P.; Akther S.; Hannan J.; Seidel V.; Nujat N. J.; Abdel-Wahab Y. H. Pharmacologically active phytomolecules isolated from traditional antidiabetic plants and their therapeutic role for the management of diabetes mellitus. Molecules 2022, 27 (13), 4278.10.3390/molecules27134278. - DOI - PMC - PubMed
-
- ElHajj Chehadeh S.; Sayed N. S.; Abdelsamad H. S.; Almahmeed W.; Khandoker A. H.; Jelinek H. F.; Alsafar H. S. Genetic variants and their associations to type 2 diabetes mellitus complications in The United Arab Emirates. Front. Endocrinol. 2022, 12, 751885.10.3389/fendo.2021.751885. - DOI - PMC - PubMed
-
- Trang Nguyen N. D.; Le L. T. Targeted proteins for diabetes drug design. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3 (1), 013001.10.1088/2043-6262/3/1/013001. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
