Infrared Free-Electron Laser: A Versatile Molecular Cutter for Analyzing Solid-State Biomacromolecules
- PMID: 40256544
- PMCID: PMC12004168
- DOI: 10.1021/acsomega.4c07531
Infrared Free-Electron Laser: A Versatile Molecular Cutter for Analyzing Solid-State Biomacromolecules
Abstract
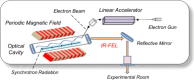
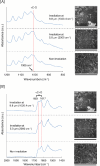
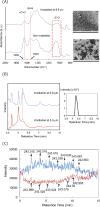
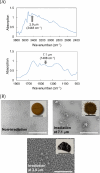
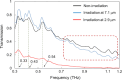
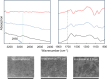
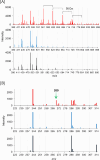
Free-electron lasers that oscillate in the infrared (IR) range of 1000 (10 μm) to 4000 cm-1 (2.5 μm) were applied to irradiate solid-phase polysaccharides and aromatic biomacromolecules. Synchrotron radiation IR microscopy (SR-IRM) and electrospray ionization mass spectroscopy (ESI-MS) analyses showed that N-acetyl glucosamine was isolated from the powdered exoskeleton of crayfish by irradiation at 1020 cm-1 (9.8 μm), resonating with the C-O stretching mode (νC-O). Irradiation at 3448 cm-1 (2.9 μm), which is resonant with the O-H stretching vibration (νO-H) of sulfonated lignin, dissociates the aggregate state and releases coniferyl aldehyde substituted with sulfinate, as shown by scanning electron microscopy, terahertz-coherent edge radiation spectroscopy, SR-IRM, and ESI-MS. These vibrational excitation reactions proceed at room temperature in the absence of solvent. Current and previous studies have demonstrated that intense IR lasers can be used as versatile tools for unveiling the internal structures of persistent biomacromolecules.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Degradation of Lignin by Infrared Free Electron Laser.Polymers (Basel). 2022 Jun 14;14(12):2401. doi: 10.3390/polym14122401. Polymers (Basel). 2022. PMID: 35745977 Free PMC article.
-
Modulations of νO-H and νC═O Stretching Frequencies of Difluoroacetic Acid with Internal Rotation of CHF2 Rotor: A Combined Vapor Phase and Matrix Isolation Infrared Spectroscopy Study.J Phys Chem A. 2019 Apr 4;123(13):2771-2779. doi: 10.1021/acs.jpca.8b12528. Epub 2019 Mar 21. J Phys Chem A. 2019. PMID: 30852897
-
State-selective vibrational excitation and dissociation of H2+ by strong infrared laser pulses: below-resonant versus resonant laser fields and electron-field following.J Phys Chem A. 2012 Nov 26;116(46):11388-97. doi: 10.1021/jp3060679. Epub 2012 Aug 31. J Phys Chem A. 2012. PMID: 22916791
-
Biological applications of synchrotron radiation infrared spectromicroscopy.Biotechnol Adv. 2012 Nov-Dec;30(6):1390-404. doi: 10.1016/j.biotechadv.2012.02.012. Epub 2012 Feb 28. Biotechnol Adv. 2012. PMID: 22401782 Review.
-
Disassembly of Amyloid Fibril with Infrared Free Electron Laser.Int J Mol Sci. 2023 Feb 12;24(4):3686. doi: 10.3390/ijms24043686. Int J Mol Sci. 2023. PMID: 36835098 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
