Enhancement of apoptosis in HCT116 and HepG2 cells by Coix lacryma-jobi var. lacryma-jobi seed extract in combination with sorafenib
- PMID: 40256710
- PMCID: PMC12009101
- DOI: 10.1016/j.chmed.2025.02.005
Enhancement of apoptosis in HCT116 and HepG2 cells by Coix lacryma-jobi var. lacryma-jobi seed extract in combination with sorafenib
Abstract
Objective: Coix lacryma-jobi, a highly regarded Asian herb widely used in traditional Chinese medicine, is recognized for its dual benefits in promoting overall health and treating various diseases. While it exhibits moderate anticancer efficacy when used alone, this study investigated the enhanced anticancer potential of raw and cooked Coix lacryma-jobi var. lacryma-jobi (CL) seed extracts in combination with sorafenib against HCT116 and HepG2 cancer cell lines. The combination of sorafenib with other anticancer agents, including natural extracts, has garnered significant attention as a promising strategy for developing more effective cancer therapies.
Methods: Dry powders of raw (R) and cooked (C) CL seeds, obtained from a local commercial source in Thailand, were extracted and fractionated using ethanol (E), dichloromethane (D), ethyl acetate (A), and water (W) to produce eight fractions: CLRE, CLCE, CLRD, CLCD, CLRA, CLCA, CLRW, and CLCW. The coixol content in raw and cooked seed extracts was quantified and expressed as μg of coixol per gram of extract. The cytotoxic effects of these fractions were evaluated against HCT116 and HepG2 cells using the MTT assay. Fractions demonstrating the most significant cytotoxic responses were combined with sorafenib to evaluate their synergistic effects. Apoptosis induction and mitochondrial membrane potential (MMP) were assessed, and the underlying mechanism of apoptosis was explored by analyzing reactive oxygen species (ROS) generation and antioxidant protein expression levels. Additionally, the combination treatment's effect on the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) pathway was investigated.
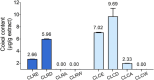
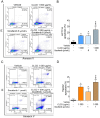
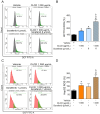
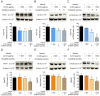
Results: One gram of CLCE and CLCD extracts contained higher coixol levels (7.02 μg and 9.69 μg, respectively) compared to CLRE and CLRD (2.66 μg and 5.96 μg, respectively). Coixol content in CLRA, CLRW, and CLCW fractions was undetectable under the study conditions. All extract fractions exhibited IC50 values exceeding 1 mg/mL after 24- and 48-hour incubations with HCT116 and HepG2 cells, indicating limited cytotoxicity when used independently. CLRD and CLCD fractions were selected for combination studies at a concentration of 1 mg/mL, combined with sub-IC50 concentrations of sorafenib to minimize its side effects. This combination significantly increased cytotoxicity, inducing apoptosis in HCT116 and HepG2 cells by elevating ROS levels and reducing the expression of superoxide dismutase 2 and catalase. Furthermore, the combination treatment downregulated the PI3K/AKT/mTOR pathway, indicating a targeted anticancer mechanism.
Conclusion: The combination of CLCD with sorafenib demonstrates significant potential as a strategy for future anticancer therapies. This CL seed extract, cultivated and commercially available in Thailand, shows promise as a natural supplement to enhance the efficacy of chemotherapy in upcoming clinical anticancer applications.
Keywords: Coix lacryma-jobi var. lacryma-jobi; HCT116; HepG2; PI3K/AKT/mTOR pathway; apoptosis; reactive oxygen species; seed extracts; sorafenib.
© 2025 Tianjin Press of Chinese Herbal Medicines. Published by ELSEVIER B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Cerbera odollam fruit extracts enhance anti-cancer activity of sorafenib in HCT116 and HepG2 cells.Chin Herb Med. 2024 Nov 20;17(1):108-126. doi: 10.1016/j.chmed.2024.11.007. eCollection 2025 Jan. Chin Herb Med. 2024. PMID: 39949813 Free PMC article.
-
Coix lacryma-jobi var. ma-yuen Stapf sprout extract induces cell cycle arrest and apoptosis in human cervical carcinoma cells.BMC Complement Altern Med. 2019 Nov 15;19(1):312. doi: 10.1186/s12906-019-2725-z. BMC Complement Altern Med. 2019. PMID: 31729992 Free PMC article.
-
Two new amides from the seeds of Coix lacryma-jobi var. lacryma-jobi.Nat Prod Res. 2023 Oct-Nov;37(20):3499-3504. doi: 10.1080/14786419.2022.2089669. Epub 2022 Jun 16. Nat Prod Res. 2023. PMID: 35707908
-
Anti-tumor effect of coix seed based on the theory of medicinal and food homology.World J Clin Oncol. 2023 Dec 24;14(12):593-605. doi: 10.5306/wjco.v14.i12.593. World J Clin Oncol. 2023. PMID: 38179404 Free PMC article. Review.
-
Research on Coix seed as a food and medicinal resource, it's chemical components and their pharmacological activities: A review.J Ethnopharmacol. 2024 Jan 30;319(Pt 3):117309. doi: 10.1016/j.jep.2023.117309. Epub 2023 Oct 17. J Ethnopharmacol. 2024. PMID: 37858750 Review.
References
-
- Afrin S., Giampieri F., Cianciosi D., Alvarez-Suarez J.M., Bullon B., Amici A.…Battino M. Strawberry tree honey in combination with 5-fluorouracil enhances chemosensitivity in human colon adenocarcinoma cells. Food and Chemical Toxicology. 2021;156 - PubMed
-
- Afrin S., Giampieri F., Forbes-Hernández T.Y., Gasparrini M., Amici A., Cianciosi D., Quiles J.L., Battino M. Manuka honey synergistically enhances the chemopreventive effect of 5-fluorouracil on human colon cancer cells by inducing oxidative stress and apoptosis, altering metabolic phenotypes and suppressing metastasis ability. Free Radical Biology and Medicine. 2018;126:41–54. - PubMed
-
- Alsulaimany M., El-Adl K., Aljohani A.K.B., Alharbi H.Y., Alatawi O.M., Aljohani M.S.…Mohamed A.A. Design, synthesis, docking, ADMET and anticancer evaluations of N-alkyl substituted iodoquinazoline derivatives as dual VEGFR-2 and EGFR inhibitors. RSC Advances. 2023;13(51):36301–36321. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

