The Release of Non-Native Gamebirds Is Associated With Amplified Zoonotic Disease Risk
- PMID: 40256856
- PMCID: PMC12010324
- DOI: 10.1111/ele.70115
The Release of Non-Native Gamebirds Is Associated With Amplified Zoonotic Disease Risk
Abstract
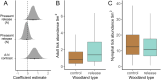
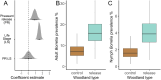
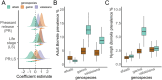
Spillback-where non-native species increase native pathogen prevalence-is potentially an important mechanism by which non-natives contribute to zoonotic disease emergence. However, spillback has not yet been directly demonstrated because it is difficult to disentangle from confounding factors which correlate with non-native species abundance and native pathogen prevalence. Here, we capitalise on replicated, quasi-experimental releases of non-native pheasants (Phasianus colchicus) to compare vector abundance and native pathogen prevalence between sites with similar local conditions but different non-native densities. Prevalence of Borrelia spp. (the causative agent of Lyme disease) in questing ticks was almost 2.5x higher in woods where pheasants are released compared to control woods, with a particularly strong effect on Borrelia garinii, a bird specialist genospecies. Furthermore, adult (but not nymphal) ticks tended to be more abundant at pheasant-release woods. This work provides evidence that non-native species can impact zoonotic pathogen prevalence via spillback in ecologically relevant contexts.
Keywords: Borrelia burgdorferi sensu lato; environmental change; invasive species; one health; spillover; zoonosis.
Ecology Letters© 2025 The Author(s). Ecology Letters published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Multilocus Sequence Typing Unveils Two Novel Genospecies of Borrelia burgdorferi Sensu Lato in Ticks Infesting Cricetid Rodents of Northern Chile.Transbound Emerg Dis. 2025 Jul 10;2025:8841276. doi: 10.1155/tbed/8841276. eCollection 2025. Transbound Emerg Dis. 2025. PMID: 40687775 Free PMC article.
-
The effect of fluralaner treatment of small mammals on the endemic cycle of Borrelia burgdorferi in a natural environment.J Med Entomol. 2024 Sep 10;61(5):1203-1213. doi: 10.1093/jme/tjae091. J Med Entomol. 2024. PMID: 39119633 Free PMC article.
-
Landscape structure affects the prevalence and distribution of a tick-borne zoonotic pathogen.Parasit Vectors. 2018 Dec 4;11(1):621. doi: 10.1186/s13071-018-3200-2. Parasit Vectors. 2018. PMID: 30514350 Free PMC article.
-
Research effort on birds' reservoir host potential for Lyme borreliosis: A systematic review and perspectives.Transbound Emerg Dis. 2022 Sep;69(5):2512-2522. doi: 10.1111/tbed.14305. Epub 2021 Sep 21. Transbound Emerg Dis. 2022. PMID: 34453490
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Aebischer, N. J. 2019. “Fifty‐Year Trends in UK Hunting Bags of Birds and Mammals, and Calibrated Estimation of National bag Size, Using GWCT'S National Gamebag Census.” European Journal of Wildlife Research 65, no. 4: 64.
-
- Baguette, M. , and Van Dyck H.. 2007. “Landscape Connectivity and Animal Behavior: Functional Grain as a Key Determinant for Dispersal.” Landscape Ecology 22: 1117–1129.
-
- Baranton, G. U. Y. , Postic D., Saint Girons I., et al. 1992. “Delineation of Borrelia Burgdorferi Sensu Stricto, Borrelia garinii sp. nov., and Group VS461 Associated With Lyme Borreliosis.” International Journal of Systematic and Evolutionary Microbiology 42, no. 3: 378–383. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

