Recommendations for the Use of Disease-Modifying Antirheumatic Drugs in Pregnancy and Reproductive Health for Patients With Rheumatic Disease: A Scoping Review
- PMID: 40256995
- PMCID: PMC12500500
- DOI: 10.1002/acr.25558
Recommendations for the Use of Disease-Modifying Antirheumatic Drugs in Pregnancy and Reproductive Health for Patients With Rheumatic Disease: A Scoping Review
Abstract
Objective: Autoimmune rheumatic diseases commonly affect individuals of childbearing age, with historically increased adverse pregnancy outcomes in this group. The advent of disease-modifying antirheumatic drugs (DMARDs) has fostered more suitable conditions for pregnancy; however, this is accompanied by challenges in ensuring safe use in reproductive health. The aim of this review is to compare existing guideline recommendations for the use of DMARDs in pregnancy and reproductive health for patients with rheumatic disease.
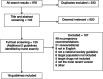
Methods: A scoping review was performed with Medline and Embase, in addition to a hand search, to identify guidelines published since 2014 by academic societies in rheumatology that addressed the management of DMARDs in pregnancy in any of rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and systemic lupus erythematosus. Conventional synthetic DMARDs (csDMARDs) (methotrexate, sulfasalazine, leflunomide, and hydroxychloroquine), biologic DMARDs (bDMARDs) (adalimumab, etanercept, infliximab, golimumab, certolizumab, abatacept, tocilizumab, rituximab, and anakinra), and targeted synthetic DMARDs (tsDMARDs) (tofacitinib, baricitinib, and upadacitinib) were targeted. Two authors performed data extraction in duplicate (AC, AT).
Results: A total of 18 guidelines were included. Recommendations for DMARD use in preconception were present in 10 guidelines (56%), lactation in 12 guidelines (67%), and male fertility in 6 guidelines (33%). A total of 13 guidelines (72%) included recommendations for csDMARDs, 13 guidelines (72%) included recommendations for bDMARDs, and 5 guidelines (28%) included recommendations for tsDMARDs. There was moderate evidence supporting relatively uniform csDMARD recommendations, compared to minimal evidence for bDMARD and tsDMARD use with variable recommendations.
Conclusion: There is heterogeneity in the formulation of guidelines on the use of DMARDs in pregnancy. Recommendations for csDMARDs were similar between guidelines. There was significant variability in recommendations for bDMARD and tsDMARD use, reflecting current minimal literature in this area.
© 2025 The Author(s). Arthritis Care & Research published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
References
-
- Sugawara E, Kato M, Fujieda Y, et al. Pregnancy outcomes in women with rheumatic diseases: a real‐world observational study in Japan. Lupus 2019;28(12):1407–1416. - PubMed
-
- Wallenius M, Salvesen KÅ, Daltveit AK, et al. Secular trends of pregnancies in women with inflammatory connective tissue disease. Acta Obstet Gynecol Scand 2015;94(11):1195–1202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials