Cognitive implications and associated transcriptomic signatures of distinct regional iron depositions in cerebral small vessel disease
- PMID: 40257048
- PMCID: PMC12010275
- DOI: 10.1002/alz.70196
Cognitive implications and associated transcriptomic signatures of distinct regional iron depositions in cerebral small vessel disease
Abstract
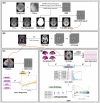
Introduction: Regional brain iron dyshomeostasis is observed in cerebral small vessel disease (cSVD) and other neurodegeneration processes. However, its spatial patterns, cognitive impact, and underlying pathological mechanisms remain unclear.
Methods: Voxel-based analysis of quantitative susceptibility mapping (QSM) was used to detect regional susceptibility changes, and their correlations with cognitive function were assessed using linear regression. We combined the microarray dataset from the Allen Human Brain Atlas (AHBA) to explore the pathological mechanisms of iron deposition patterns.
Results: A total of 87 cSVD patients and 80 controls were included in the study. Increased QSM values in the bilateral putamen and caudate were associated with cognitive decline in cSVD. Gene set enrichment analysis revealed the enrichment of gene sets related to central nervous system integrity.
Discussion: Iron deposition in deep gray matter may indicate cognitive changes in cSVD and could be linked to the disruption of brain structural and functional integrity.
Highlights: Increased susceptibility values, indicating focal iron deposition, were observed in the deep gray matter of patients with cerebral small vessel disease (cSVD). Regional iron concentration in the deep gray nuclei was associated with cognitive impairment in cSVD patients. Imaging transcriptomics suggests that cSVD-related iron deposition is linked to the structural and functional integrity of the brain. An open-source script for imaging transcriptomics focusing on regional gene expression was developed and proposed.
Keywords: Allen Human Brain Atlas; cerebral small vessel disease; gene expression; iron deposition; quantitative susceptibility mapping.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflict of interest. Author disclosures are available in the supporting information.
Figures



Similar articles
-
Brain iron deposition and cognitive decline in patients with cerebral small vessel disease : a quantitative susceptibility mapping study.Alzheimers Res Ther. 2025 Jan 9;17(1):17. doi: 10.1186/s13195-024-01638-x. Alzheimers Res Ther. 2025. PMID: 39789638 Free PMC article.
-
Microstructural changes in the caudate nucleus and hippocampus and their association with cognitive function in cerebral small vessel disease: A quantitative susceptibility mapping study.Neurobiol Dis. 2025 Aug;212:106964. doi: 10.1016/j.nbd.2025.106964. Epub 2025 May 20. Neurobiol Dis. 2025. PMID: 40404061
-
Cerebral Microbleeds Are Associated With Increased Brain Iron and Cognitive Impairment in Patients With Cerebral Small Vessel Disease: A Quantitative Susceptibility Mapping Study.J Magn Reson Imaging. 2022 Sep;56(3):904-914. doi: 10.1002/jmri.28092. Epub 2022 Jan 31. J Magn Reson Imaging. 2022. PMID: 35099829
-
Quantitative susceptibility mapping of brain iron in healthy aging and cognition.Neuroimage. 2023 Nov 15;282:120401. doi: 10.1016/j.neuroimage.2023.120401. Epub 2023 Oct 5. Neuroimage. 2023. PMID: 37802405 Free PMC article. Review.
-
Neuroimaging studies on cognitive impairment due to cerebral small vessel disease.Stroke Vasc Neurol. 2019 Apr 5;4(2):99-101. doi: 10.1136/svn-2018-000209. eCollection 2019 Jul. Stroke Vasc Neurol. 2019. PMID: 31338220 Free PMC article. Review.
Cited by
-
Data Mining and Biochemical Profiling Reveal Novel Biomarker Candidates in Alzheimer's Disease.Int J Mol Sci. 2025 Aug 4;26(15):7536. doi: 10.3390/ijms26157536. Int J Mol Sci. 2025. PMID: 40806665 Free PMC article.
References
-
- Duering M, Biessels GJ, Brodtmann A, et al. Neuroimaging standards for research into small vessel disease—advances since 2013. Lancet Neurol. 2023;22:602‐618. - PubMed
MeSH terms
Substances
Grants and funding
- 2024HXBH023/Post Doctor Research Fund of West China Hospital, Sichuan University
- GZC20241151/Postdoctoral Fellowship Program of CPSF
- Medical- Engineering Integration Interdisciplinary Talent
- HXDZ22011/ZYGX2022YGRH017/University of Electronic Science and Technology of China
- 2024-F05-00541-SN/Technology Innovation R&D Project of Chengdu Science and Technology Bureau
- 82071320/National Natural Science Foundation of China
- 82371322/National Natural Science Foundation of China
- U24A20690/Joint Funds of the National Natural Science Foundation of China
- 2023YFC2506603/National Key R&D Program of China
- 2023ZD0504900/Noncommunicable Chronic Diseases-National Science and Technology Major Project
LinkOut - more resources
Full Text Sources
Medical

