Artificial Intelligence: Applications in Pharmacovigilance Signal Management
- PMID: 40257538
- PMCID: PMC12126317
- DOI: 10.1007/s40290-025-00561-2
Artificial Intelligence: Applications in Pharmacovigilance Signal Management
Abstract
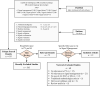
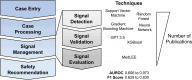
Pharmacovigilance is the science of collection, detection, and assessment of adverse events associated with pharmaceutical products for the ongoing monitoring and understanding of those products' safety profiles. Part of this process, signal management, encompasses the activities of signal detection, signal validation/confirmation, signal evaluation, and ultimately, final assessment as to whether a safety signal constitutes a new causal adverse drug reaction. Artificial intelligence is a group of technologies including machine learning and natural language processing that are revolutionizing multiple industries through intelligent automation. Here, we present a critical evaluation of studies leveraging artificial intelligence in signal management to characterize the benefits and limitations of the technology, the level of transparency, and our perspective on best practices for the future. To this end, PubMed and Embase were searched cumulatively for terms pertaining to signal management and artificial intelligence, machine learning, or natural language processing. Information pertaining to the artificial intelligence model used, hyperparameter settings, training/testing data, performance, feature analysis, and more was extracted from included articles. Common signal detection methods included k-means, random forest, and gradient boosting machine. Machine learning algorithms generally outperformed traditional frequentist or Bayesian measures of disproportionality per various metrics, showing the potential utility of advanced machine learning technologies in signal detection. In signal validation and evaluation, natural language processing was typically applied. Overall, methodological transparency was mixed and only some studies leveraged "gold standard" publicly available positive and negative control datasets. Overall, innovation in pharmacovigilance signal management is being driven by machine learning and natural language processing models, particularly in signal detection, in part because of high-performing bagging methods such as random forest and gradient boosting machine. These technologies may be well poised to accelerate progress in this field when used transparently and ethically. Future research is needed to assess the applicability of these techniques across various therapeutic areas and drug classes in the broader pharmaceutical industry.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: The authors did not receive any funds from any funding agency for the preparation of this article. Conflict of interest: Jeffrey Warner, Anaclara Prada Jardim, and Claudia Albera are employees of Eli Lilly and Company. Anaclara Prada Jardim and Claudia Albera are shareholders in Eli Lilly and Company. Funding for this work was provided by Eli Lilly and Company. Ethics approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Availability of data and material: Data are available from the corresponding author upon reasonable request. Code availability: Not applicable. Author contributions: Conceptualization: JW, AJ, CA; literature search and data analysis: JW; writing (original draft preparation): JW; writing (review and editing): JW, AJ, CA; supervision: CA. All authors have read and approved the final submitted manuscript and agree to be accountable for this work.
Figures

References
-
- European Medicines Agency. EMA/95595/2022: guidelines on good pharmacovigilance practices (GVP). In: European Medicines Agency, editor. Introductory cover note, last updated with release of Addendum III of Module XVI on pregnancy prevention programmes for public consultation. Amsterdam: Heads of Medicines Agencies; 2022. p. 4.
-
- World Health Organization. Regulation and prequalification: what is pharmacovigilance? Available from: https://www.who.int/teams/regulation-prequalification/regulation-and-saf.... Accessed 6 Sep 2024.
-
- European Medicines Agency. EMA/827661/2011: guideline on good pharmacovigilance practices (GVP). In: European Medicines Agency, editor. Module IX: signal management (Rev 1). Amsterdam: Heads of Medicines Agencies; 2017.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

