Dysregulation of cholesterol homeostasis in cancer pathogenesis
- PMID: 40257622
- PMCID: PMC12011706
- DOI: 10.1007/s00018-025-05617-9
Dysregulation of cholesterol homeostasis in cancer pathogenesis
Abstract
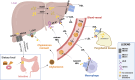
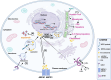
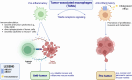
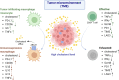
Cholesterol is a unique lipid for all mammalian cells, with important functions in membrane biogenesis and maintenance of proper membrane integrity and fluidity. Therefore, it plays an important role in cellular homeostasis. Dysregulation of cholesterol homeostasis is associated with various diseases in humans, including cardiovascular diseases, inflammatory diseases, neurodegenerative disorders, and cancers. In the tumor microenvironment, intrinsic and extrinsic cellular factors reprogram cholesterol metabolism and consequently promote tumorigenesis. Here, we summarize the current knowledge on molecular mechanisms and functional roles of cholesterol homeostasis and its dysregulation in regard to cancer pathogenesis. We also discuss the interplay of cholesterol metabolism and the ATP-binding cassette (ABC) proteins, highly conserved cellular transmembrane lipid transporters. An emerging role of lipid ABC transporters as potential prognostic tools for cancer progression and invasiveness is emphasized. Targeting both cholesterol metabolism and proteins associated with membrane cholesterol holds promise as a novel therapeutic strategy for cancer treatment.
Keywords: ATP-binding cassette transporters; Cancer; Cancer progression; Cholesterol metabolism; Membrane cholesterol; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no financial or non-financial interests to disclose.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

