Cytisinicline for Smoking Cessation: The ORCA Phase 3 Replication Randomized Clinical Trial
- PMID: 40257755
- PMCID: PMC12012700
- DOI: 10.1001/jamainternmed.2025.0628
Cytisinicline for Smoking Cessation: The ORCA Phase 3 Replication Randomized Clinical Trial
Abstract
Importance: New smoking cessation medication options are needed. Cytisinicline, a partial agonist at α4β2 nicotinic acetylcholine receptors, has demonstrated smoking cessation efficacy in 1 US trial. Additional evidence is needed.
Objective: To reproduce the findings of the efficacy and tolerability of cytisinicline compared with placebo for smoking cessation and to test its effect on nicotine craving as a mechanism of action.
Design, settings, and participants: This was a 3-group double-blind, placebo-controlled phase 3 replication randomized clinical trial (ORCA-3) conducted at 20 clinical trial sites in the US from January 2022 to March 2023. It compared 6 and 12 weeks of a novel cytisinicline regimen to placebo among adults who smoked 10 or more cigarettes daily and sought to quit. Participants were randomized (1:1:1) to 3-mg cytisinicline 3 times daily for 12 weeks; 3-mg cytisinicline 3 times daily for 6 weeks followed by placebo for 6 weeks; or placebo 3 times daily for 12 weeks. The follow-up period was 24 weeks, and all groups received behavioral support. Data analyses were performed from May 3, 2023, to March 20, 2024.
Interventions: Cytisinicline, 3 mg, 3 times daily for 12 weeks; cytisinicline, 3 mg, 3 times daily for 6 weeks followed by placebo for 6 weeks; or placebo 3 times daily for 12 weeks.
Main outcomes and measures: Biochemically verified (carbon monoxide <10 ppm) continuous smoking abstinence during the last 4 weeks of 6- and 12-week treatments (primary outcome) and from end of treatment to 24 weeks (secondary outcome); Questionnaire of Smoking Urges; incidence of adverse events.
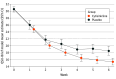
Results: Of 792 participants randomized (mean [SD] age, 52.0 [11.8] years; 439 [55.4%] female; mean [SD] cigarettes/d, 20.4 [7.5]), 628 (79.3%) completed the trial. Primary and secondary outcomes were significantly higher for both cytisinicline groups vs placebo. For 6-week treatment, 39 cytisinicline participants (14.8%) vs 16 placebo participants (6.0%) were abstinent during weeks 3 to 6 (odds ratio [OR], 2.9; 95% CI, 1.5-5.6; P < .001). For 12-week treatment, 80 cytisinicline participants (30.3%) vs 25 placebo participants (9.4%) were abstinent during weeks 9 to 12 (OR, 4.4; 95% CI, 2.6-7.3; P < .001). Continuous abstinence rates for the 6-week treatment were 6.8% (cytisinicline) vs 1.1% (placebo) from weeks 3 to 24 . Continuous abstinence rates for the 12-week treatment were 20.5% (cytisinicline) vs 4.2% (placebo) for weeks 9 to 24. Reduction in craving at week 6 was greater for cytisinicline than placebo (-15.2 points [95% CI, -16.4 to -14.0] vs -12.0 points [95% CI, -13.5 to -10.5]; P < .001). Cytisinicline was well tolerated with no treatment-related serious adverse events.
Conclusions and relevance: The findings of the ORCA phase 3 trial reaffirms the efficacy and tolerability of cytisinicline at both 6- and 12-week treatment for smoking cessation, with benefits extending through 24 weeks. As a mechanism of effect, cytisinicline mitigated nicotine craving.
Trial registration: ClinicalTrials.gov Identifier: NCT05206370.
Conflict of interest statement
Figures

References
-
- World Health Organization . WHO report on the global tobacco epidemic, 2019: monitoring tobacco use and prevention policies. Geneva; 2019.
-
- US Department of Health and Human Services . Smoking Cessation: A Report of the Surgeon General— Executive Summary. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020.
-
- Patnode CD, Henderson JT, Coppola EL, Melnikow J, Durbin S, Thomas RG. Interventions for tobacco cessation in adults, including pregnant persons: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(3):280-298. doi:10.1001/jama.2020.23541 - DOI - PubMed
-
- Anthenelli RM, Benowitz NL, West R, et al. . Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

