Detailed Clinical, Ophthalmic, and Genetic Characterization of MYO7A-Associated Usher Syndrome
- PMID: 40257781
- PMCID: PMC12020961
- DOI: 10.1167/iovs.66.4.60
Detailed Clinical, Ophthalmic, and Genetic Characterization of MYO7A-Associated Usher Syndrome
Abstract
Purpose: To analyze the clinical spectrum and natural history of MYO7A-associated Usher syndrome type I (USH1).
Methods: Patients with molecularly confirmed MYO7A-associated USH1 in a single tertiary referral center. Data was extracted from physical and electronic case notes, including imaging and electrophysiology. Genetic results were reviewed, and the detected variants were assessed. Main outcome measures were clinical findings, qualitative and quantitative analysis of retinal imaging, and electrophysiology.
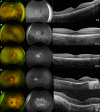
Results: Eighty patients were identified and evaluated longitudinally. The mean age (±SD) of onset of symptoms was 12.0 ± 5.8 years of age, and a mean follow-up time of 16.2 years. BCVA was 0.4 ± 0.5 LogMAR at baseline, and 0.7 ± 0.8 LogMAR at the last visit for both eyes. The change in BCVA over time was 0.02 LogMAR per year. A hyperautofluorescent (hyperAF) ring was present in 51% of the patients. The mean ellipsoid zone width (EZW) at baseline was 2568.2 ± 1528.9 µm OD and 2527.9 ± 1609.3 µm OS, which decreased to 2012.3 ± 1705.1 µm OD and 1806.3 ± 1647.1 µm OS at last visit. Electrophysiology revealed rod and cone dysfunction with relative or complete sparing of macular function. There were statistically significant changes in BCVA, EZW, and hyperAF ring between baseline and follow-up. Genetic analysis identified 83 variants in MYO7A, including 18 novel variants.
Conclusions: Longitudinal analysis shows that the majority of patients retain central visual function and structure until the fifth decade of life, which informs advice on prognosis and the window for therapeutic intervention.
Conflict of interest statement
Disclosure:
Figures






References
-
- Boughman JA, Vernon M, Shaver KA.. Usher syndrome: Definition and estimate of prevalence from two high-risk populations. J Chronic Dis. 1983; 36: 595–603. - PubMed
-
- Ullah F, Zeeshan Ali M, Ahmad S, et al. .. Current updates on genetic spectrum of usher syndrome. Nucleosides Nucleotides Nucleic Acids. 2025; 44: 337–360. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

