Oral vs Extended-Release Injectable Naltrexone for Hospitalized Patients With Alcohol Use Disorder: A Randomized Clinical Trial
- PMID: 40257810
- PMCID: PMC12013356
- DOI: 10.1001/jamainternmed.2025.0522
Oral vs Extended-Release Injectable Naltrexone for Hospitalized Patients With Alcohol Use Disorder: A Randomized Clinical Trial
Abstract
Importance: Alcohol use disorder (AUD) is common in hospital patients. AUD medications are not typically initiated in that setting. The comparative effectiveness between initiation of oral naltrexone and extended-release injectable naltrexone in the hospital is not known.
Objective: To compare the effectiveness of initiating oral naltrexone vs extended-release injectable naltrexone on reduction in alcohol use and health care utilization among medical inpatients with AUD.
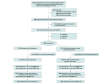
Design, setting, and participants: The Alcohol Disorder Hospital Treatment (ADOPT) study is a randomized clinical trial conducted at an urban teaching hospital in the US, with enrollment between June 2016 and March 2020. Inpatients were screened for eligibility, and those with AUD and recent heavy drinking (defined as 5 or more drinks for men and 4 or more drinks for women) were enrolled. Outcomes were assessed at 3-month follow-up; assessors were not blinded to treatment assignment. Data were analyzed from May 2021 to September 2023.
Interventions: Participants received either daily oral naltrexone or monthly extended-release injectable naltrexone. All received medical management with a research nurse who specialized in addiction.
Main measures and outcomes: The primary outcome was change in percentage of heavy drinking days (HDDs) over the past 30 days from baseline to 3-month follow-up, assessed by validated instrument. The secondary outcome was any acute health care utilization (emergency department or hospitalization) at 3-month follow-up over the past 90 days.
Results: Of 248 participants, 199 (80.2%) were male, and the mean (SD) age was 49.4 (10.4) years. The baseline median (IQR) percentage of HDDs in the past 30 days was 80.0% (43.3-100). At 3-month follow-up, the mean percentage of HDDs in the past 30 days was reduced in both groups (oral naltrexone: baseline, 66.7% HDDs; 3-month follow-up, 27.4% HDDs; difference, -38.4 percentage points; 95% CI, -125.0 to 48.2; extended-release injectable naltrexone: baseline, 70.7% HDDs; 3-month follow-up, 23.8% HDDs; difference, -46.4 percentage points; 95% CI, -123.4 to 30.6; P = .14). At follow-up, 59 of 109 in the oral naltrexone arm (54.1%) and 66 of 108 in the extended-release injectable naltrexone arm (61.1%) reported acute health care utilization in the prior 3 months; the odds of this utilization were not significantly different between groups (adjusted odds ratio, 1.34; 95% CI, 0.77-2.33).
Conclusions and relevance: In this randomized clinical trial, when initiated at hospital discharge, oral and extended-release injectable naltrexone did not differ in effectiveness. Participants had substantial reductions in HDDs in both treatment groups; however, there was not a significant difference in the reduction of percentage of HDDs in the past 30 days or acute health care utilization between groups. Hospitalization represents an opportunity to start AUD pharmacotherapy; choice of oral naltrexone vs extended-release injectable naltrexone should be directed by factors such as patient preference and insurance.
Trial registration: ClinicalTrials.gov Identifier: NCT02478489.
Conflict of interest statement
Figures

Comment on
-
Hospital-Initiated Naltrexone for Alcohol Use Disorder.JAMA Intern Med. 2025 Jun 1;185(6):645-647. doi: 10.1001/jamainternmed.2025.0532. JAMA Intern Med. 2025. PMID: 40257770 No abstract available.
Similar articles
-
Predischarge Injectable Versus Oral Naltrexone to Improve Postdischarge Treatment Engagement Among Hospitalized Veterans with Alcohol Use Disorder: A Randomized Pilot Proof-of-Concept Study.Alcohol Clin Exp Res. 2017 Jul;41(7):1352-1360. doi: 10.1111/acer.13410. Epub 2017 Jun 12. Alcohol Clin Exp Res. 2017. PMID: 28605827 Free PMC article. Clinical Trial.
-
Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence: A Randomized Clinical Noninferiority Trial.JAMA Psychiatry. 2017 Dec 1;74(12):1197-1205. doi: 10.1001/jamapsychiatry.2017.3206. JAMA Psychiatry. 2017. PMID: 29049469 Free PMC article. Clinical Trial.
-
Extended-release vs. oral naltrexone for alcohol dependence treatment in primary care (XON).Contemp Clin Trials. 2019 Jun;81:102-109. doi: 10.1016/j.cct.2019.04.006. Epub 2019 Apr 12. Contemp Clin Trials. 2019. PMID: 30986535 Free PMC article. Clinical Trial.
-
Long-acting injectable naltrexone for the treatment of alcohol dependence.Expert Rev Neurother. 2007 Oct;7(10):1265-77. doi: 10.1586/14737175.7.10.1265. Expert Rev Neurother. 2007. PMID: 17939765 Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
References
-
- Chen CM, Yi HY, Falk DE, Stinson FS, Dawson DA, Grant BF. Alcohol use and alcohol use disorders in the United States: main findings from the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Accessed October 13, 2022. https://www.niaaa.nih.gov/sites/default/files/NESARCDRM.pdf
-
- Substance Abuse and Mental Health Services Administration . Results from the 2011. National Survey on Drug Use and Health: summary of national findings. Accessed October 13, 2022. https://www.samhsa.gov/data/sites/default/files/Revised2k11NSDUHSummNatF...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

