Impact of Macrolide Resistance on Azithromycin for Prevention of Rehospitalization or Death Among Children Discharged From Hospitals in Western Kenya
- PMID: 40257829
- PMCID: PMC12349937
- DOI: 10.1093/infdis/jiaf208
Impact of Macrolide Resistance on Azithromycin for Prevention of Rehospitalization or Death Among Children Discharged From Hospitals in Western Kenya
Abstract
Background: The Toto Bora trial tested whether a 5-day course of azithromycin reduced the risk of rehospitalization or death in the 6 months following hospitalization among Kenyan children and found no overall benefit. We hypothesized that macrolide resistance in gut microbes could modify azithromycin's effect.
Methods: From June 2016 to November 2019, Kenyan children aged 1-59 months were enrolled at hospital discharge and randomized to azithromycin or placebo. DNA from fecal samples and Escherichia coli isolates was analyzed for common macrolide resistance genes. Cox proportional hazards regression models, including interaction terms between randomization arm and individual macrolide resistance genes, were used to analyze time to rehospitalization or death, with Bonferroni correction applied to account for multiple comparisons.
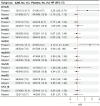
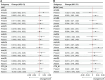
Results: Among 1393 children tested, 94.7% had at least 1 macrolide resistance gene in their fecal DNA at hospital discharge, most commonly mph(A) (68.6% [955/1393]), followed by msr(D) (67.3% [937/1393]) and erm(B) (60.7% [846/1393]). Mef(A) (23.7% [330/1393]) was the only macrolide resistance gene that modified azithromycin's effect on rehospitalization or death (interaction P = .008). In children without the mef(A) gene, azithromycin reduced the hazard of rehospitalization or death by a third (hazard ratio [HR], 0.66 [95% confidence interval {CI}, .45-.99]) whereas among children with the mef(A) gene, there was a higher risk in those randomized to azithromycin (HR, 2.72 [95% CI, 1.21-6.09]). The effect size of azithromycin's impact on mortality and rehospitalization as separate outcomes in children with and without mef(A) were consistent but underpowered.
Conclusions: Macrolide resistance in the gut microbiome may influence the efficacy of azithromycin in children discharged from the hospital. Clinical Trials Registration. NCT02414399.
Keywords: azithromycin; gut microbiome; hospital; macrolide resistance; mortality; postdischarge.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. F. C. F. is a consultant for bioMérieux, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- World Health Organization (WHO) . WHO guideline on mass drug administration of azithromycin to children under five years of age to promote child survival. Geneva, Switzerland: WHO, 2020. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

